INTRODUCTION
Globally, an estimated 180 million people are infected with the hepatitis C virus (HCV), with an estimated 3–4 million new infections each year [1]. In developed countries, the primary at-risk population for HCV infection are people who inject drugs (PWID) [1, Reference Shepard, Finelli and Alter2], with other related factors such as incarceration and homelessness [Reference Galea and Vlahov3] contributing further to HCV risk. Recently, HCV transmissions in Europe, USA, UK, Canada, and Australia have been reported in HIV-infected men who have sex with men who did not report injecting drug use, suggesting that sexual risk practices in this population may make a contribution, albeit small, to HCV transmissions in developed countries [Reference van de Laar4, Reference Gamage5]. In developing countries, in addition to injecting drug use, unsafe medical injections and blood transfusions still account for a significant proportion of newly acquired HCV infections [Reference Shepard, Finelli and Alter2].
In 2010, the World Health Organization (WHO) adopted a resolution calling for comprehensive prevention and control of viral hepatitis [6]. Surveillance of HCV is recommended by the WHO; however, the chaotic lifestyle of many PWID and non-disclosure of risk practices stemming from stigmatizing attitudes towards PWID mean that this at-risk group are often not tested for HCV, or not tested in a timely fashion [Reference Zickmund7–11]. Furthermore, newly acquired HCV is symptomatic in only about 15% of cases [Reference Maheshwari, Ray and Thuluvath12]. As a result, very few cases are detected in the early stages, with the majority detected after the patient has either cleared their infection spontaneously or progressed to chronicity [Reference Seeff13]. Furthermore, there is no incidence assay for HCV, making it difficult to identify newly acquired infection unless the patient has symptoms of acute HCV or has previously tested negative for anti-HCV antibodies.
While acknowledging the difficulty of monitoring newly acquired HCV infection and the challenges associated with reaching PWID, robust surveillance of HCV, including the ability to detect newly acquired infection, has major public health implications. Monitoring newly acquired infection is important for identifying changes in transmission rates, identifying patterns of infection, detecting outbreaks, providing the capacity to evaluate the effectiveness of public health interventions, and for developing projections of the burden of disease [Reference Buehler, Rothman and Greenland14, 15]. In addition, recent HCV treatment studies show that patients treated in the early stage of their infection are more likely to attain a sustained virological response than those who are treated in the chronic stages [Reference Kamal16–Reference Dore19]. In addition to the potential benefits for individual patients, timely treatment of PWID has potential public health benefits for the prevention of onward transmission [Reference Martin20–Reference Vickerman, Martin and Hickman24]. Thus, identifying newly acquired infections has implications for individual case management and for reducing future burden of disease.
In light of the aforementioned challenges, we conducted a literature review of methodologies for identifying newly acquired cases of HCV through HCV surveillance. The aim was to identify reports on existing surveillance systems and surveillance pilots that specify methodologies for monitoring newly acquired cases of HCV; and to evaluate the strengths and weaknesses of these surveillance methodologies in order to inform and improve surveillance of newly acquired HCV in the future.
METHODS
Investigators conducted searches between February 2009 and March 2011 using various combinations of the terms; hepatitis C, chronic hepatitis C, acute hepatitis C, hepatitis C virus, population surveillance, sentinel surveillance, enhanced surveillance, disease notification, mandatory reporting, and registries. Searches were conducted using Ovid Medline 1996 to present with daily update (http://www.ovid.com/site/catalog/DataBase/901.jsp) and ISI Web of Science (www.isiknowledge.com). Additional peer-reviewed and non-peer-reviewed literature were identified by searching the bibliographies of articles identified in these initial searches, and through searches of government websites. Literature describing methodologies of HCV surveillance systems or pilots for surveillance systems were included in the review if they discussed surveillance methodologies for identifying newly acquired cases of HCV. Pertinent definitions of HCV surveillance and classifications of surveillance methodologies are outlined in Table 1.
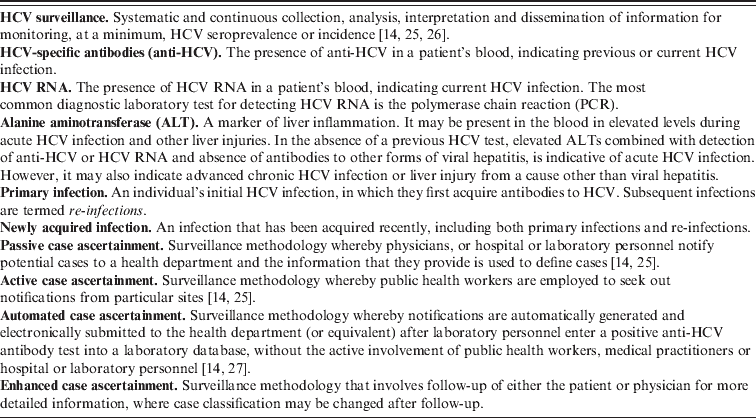
Table 1. Key definitions
RESULTS
Literature were identified describing surveillance methodologies for monitoring newly acquired cases of HCV in 15 countries [Reference Overhage, Grannis and McDonald27–Reference Tweed56]. A further 20 surveillance systems operating in these countries, and additional surveillance systems operating in 21 other countries were excluded because they did not distinguish between newly acquired and other cases of HCV [28, Reference Strauss and Bigham57–Reference Ximenes79].
In the absence of a specific HCV incidence assay being available, case definitions and case-ascertainment methodologies for monitoring newly acquired HCV differed between surveillance systems. None of the literature explicitly discussed HCV re-infection. Therefore, it is likely that case definitions for newly acquired infection were designed for identifying newly acquired primary HCV infection only. Cases were typically classified as newly acquired using one or more of the following criteria:
-
(a) physician classified – no formal case definition;
-
(b) clinical symptoms consistent with newly acquired viral hepatitis combined with laboratory confirmation of anti-HCV antibodies and/or HCV RNA;
-
(c) clinical symptoms consistent with newly acquired viral hepatitis or elevated liver function test scores, combined with laboratory confirmation of anti-HCV antibodies and/or HCV RNA;
-
(d) documented anti-HCV antibody seroconversion within a particular time-frame (the time-frames used ranged from 6 months to 2 years).
These criteria are discussed in detail below.
Criterion (a): Physician classified – no formal case definition
Only one published study has evaluated the accuracy of physicians' classifications of newly acquired infections. Physicians who notified HCV cases in the Australian state of New South Wales between August 1996 and August 1997 were asked to classify cases as newly acquired HCV on the basis of either a previous recent negative anti-HCV antibody test or clinical signs of newly acquired HCV. Physicians' classifications were evaluated through medical record review. Forty-two percent of 54 cases that were classified as newly acquired by physicians were found not to be newly acquired during the validation process, underlining the challenging task facing physicians to accurately classify newly acquired HCV infections [Reference Staff33].
Criterion (b): Clinical symptoms consistent with newly acquired viral hepatitis combined with laboratory confirmation of anti-HCV antibodies and/or HCV RNA
Case definitions for surveillance of viral hepatitis recommended by the WHO, and official bodies in the USA and in the European Union until 2008, have traditionally focused exclusively on symptomatic newly acquired HCV [15, 80, 81]. This case definition is still used in a number of surveillance systems operating in jurisdictions in the USA, in European countries such as Denmark, Hungary, Portugal and Spain, as well as some other countries such as Saudi Arabia [Reference Overhage, Grannis and McDonald27, 28, Reference Olmsted34, Reference Sokol35, Reference Spada45–Reference Mele47, Reference Memish, Knawy and El-Saed54]. Our literature search found that no formal evaluation of this method has been undertaken; however, this method is likely to underreport newly acquired infection as most newly acquired HCV cases are asymptomatic [Reference Maheshwari, Ray and Thuluvath12]. Furthermore, the subset reported may not be representative of all HCV cases because symptomatic patients may have different transmission routes [Reference Haley and Fischer82] and a different natural history to asymptomatic patients, with clearance of infection being more likely in symptomatic patients [Reference Maheshwari, Ray and Thuluvath12]. Moreover, collecting clinical information is challenging and newly diagnosed chronic cases may be misclassified as newly acquired cases if clinical information is not collected. An investigation of an observed increase in HCV notifications after the introduction of laboratory testing for HCV-specific antibodies in the USA found that about half the jurisdictions accepted cases on the basis of laboratory reports alone, and discrete dates of onset of symptoms were required by only 36% of counties, leading to artificial increases in newly acquired HCV case notifications [Reference Olmsted34].
Criterion (c): Clinical symptoms consistent with newly acquired viral hepatitis or elevated liver function test scores, combined with laboratory confirmation of anti-HCV antibodies and/or HCV RNA
One variation on surveillance of newly acquired symptomatic infection that allows for the inclusion of some asymptomatic cases is adopting a case definition that includes liver function test scores alongside anti-HCV antibody and/or HCV RNA testing. This method defines a laboratory-confirmed case as newly acquired if there are clinical signs or elevated liver function test results. Bulgaria, Greece, The Netherlands, and Egypt utilize the latter case definition in their surveillance systems [28, Reference Talaat29]. This variation allows asymptomatic cases to be reported if there are elevated liver function test results. Although no studies have evaluated the impact of using this case definition, compared to definitions that require cases to be symptomatic for inclusion, it is likely that a greater proportion of true newly acquired cases may be captured using this definition. There is, however, a risk that some people with late-stage chronic HCV may be erroneously included if their liver function is elevated [Reference Pradat83].
Criterion (d): Documented anti-HCV antibody seroconversion within a particular time-frame (the time-frames used ranged from 6 months to 2 years)
Another method of defining newly acquired cases that does not limit case ascertainment to symptomatic cases is to identify cases with evidence of anti-HCV seroconversion. That is, cases are defined as people with a negative anti-HCV antibody test, followed by a subsequent positive anti-HCV antibody test within a specified time period. Australian, Canadian, Swedish and UK surveillance systems have monitored newly acquired infection through collecting evidence of recent seroconversion, where recent was defined as within 6 months (Sweden), 1 year (Canada), 2 years (Australia), or was not defined (UK) [28, Reference Selvey30–Reference Staff33, Reference elSaadany, Gully and Giulivi40–Reference Zou42, Reference Brant55, Reference Tweed56]. In an Australian enhanced surveillance system, in which cases with demonstrated antibody seroconversion within 2 years were classified as newly acquired, 70% of newly acquired infections identified over an 18-month period were asymptomatic. This is the highest reported proportion of asymptomatic newly acquired infections identified in any surveillance system reviewed here [Reference Maheshwari, Ray and Thuluvath12, Reference Guy32], and underlines the limitations associated with monitoring newly acquired infections using symptomatic diagnostic presentations alone. In some surveillance systems, a combination of case definitions are used to capture symptomatic cases as well as cases with prior negative tests [28].
Case-ascertainment methodologies
While passive surveillance continues to be the dominant method of surveillance in many countries, other methods have also been used, including a range of enhanced and automated methods, to identify newly acquired cases (case-ascertainment methodologies are defined in Table 1). A recent evaluation of newly acquired HCV surveillance in the USA, where cases were defined on the basis of clinical and laboratory data, found that data on clinical symptoms were available for 98% of cases in enhanced surveillance systems compared to 63% of cases in passive surveillance. Where enhanced surveillance systems operated in the same jurisdictions as passive systems, 22% of cases identified by enhanced surveillance were not identified through passive surveillance [51].
In Australia and Canada, the majority of systems that monitored newly acquired HCV were enhanced surveillance systems, in which evidence of prior negative HCV-specific antibody tests or records of clinical newly acquired infection were requested through contact with notifying physicians or laboratories. A number of different enhanced surveillance methods for identifying and/or confirming newly acquired cases with evidence of anti-HCV antibody seroconversion have been implemented or trialled in these countries. These methods include systems that collect additional information on (i) all cases [Reference Selvey30, Reference Wu41, Reference Zou42], (ii) a random subset of cases [Reference Tobin84], and (iii) specific subsets of cases targeted as likely new infections [Reference Guy32]. In the latter surveillance system, cases were targeted for follow-up if they had been nominated as newly acquired by the physician in the original notification, had clinical or laboratory indicators of newly acquired infection, or were aged 16–19 years. Two pilots in the Australian state of Victoria selected similar proportions of all notifications for follow-up; the first used random selection (10% of cases selected), and the second used targeted follow-up (9% of cases selected). A greater proportion of all notified cases were classified as newly acquired using targeted follow-up (3%) compared to follow-up of randomly selected cases (1%), suggesting that the former methodology is more efficient for identifying newly acquired cases. When random selection was used, almost 80% of cases that were followed up could neither be classified as newly acquired nor persistent chronic, based on the available information. A third Australian pilot followed up all notified cases; when all notified cases were followed up, 4% of cases were classified as newly acquired [Reference Guy32, Reference Tobin84]. Similarly, a Canadian surveillance system that followed up all notified cases classified 4% of confirmed cases as newly acquired [Reference Zou42]. The Canadian system, the Australian pilot that followed up all notified cases, and the Australian pilot that selected a targeted group of cases for follow-up, only classified cases as newly acquired or other [Reference Guy32, Reference Zou42, Reference Tobin84].
While enhanced surveillance is useful for collecting detailed clinical and/or prior testing data, this method is expensive. An alternative approach for monitoring newly acquired HCV infection is to use automated systems. A study in the USA found that linking laboratory data from major testing laboratories with medical records captured 96% of unique newly acquired HCV diagnoses where cases were defined using combined clinical and laboratory data [Reference Overhage, Grannis and McDonald27]. Automated systems have also been used for collecting testing history for individual patients and in this way, identifying seroconversions. In British Columbia, Canada, a single laboratory is responsible for all confirmatory HCV testing. As a result, the laboratory database was able to be used to identify individuals with evidence of seroconversion within a specified period (5·6 new infections per 100 000 population were identified in 2005) [Reference Kuo58]. In the UK, a linked-laboratory surveillance system extracted test results from 20 public health and hospital laboratories and linked them using patient clinic number, date of birth, sex and, when available, soundex (the sound of the name). This system was able to identify repeat HCV tests in 14% of the 12 314 individuals who were tested for HCV at sexual health clinics between 2002 and 2007, and in those individuals, 80 anti-HCV seroconversions were confirmed (the testing intervals were not reported). Of the 58 144 individuals who were tested for HCV in four former public health laboratories and four public hospitals between 2002 and 2003, 10% had repeat tests, and in those individuals 23 anti-HCV seroconversions were confirmed (the median test interval was 5 months) [Reference Brant55, Reference Tweed56].
DISCUSSION
Despite the public health importance of monitoring newly acquired cases of HCV using surveillance, there was very little literature in this area. The available literature suggests that a considerable number of developed countries lack surveillance systems with the capacity to detect newly acquired infection and that those surveillance systems that do seek to detect newly acquired infection are limited in their capacity to do so. We identified surveillance systems or pilots for surveillance systems that could identify newly acquired infection in only 15 countries. In the most part, the methods used in these systems provided unreliable estimates of the true incidence of newly acquired HCV. In addition, there were a considerable number of countries without any surveillance systems capable of identifying newly acquired infection. This demonstrates that distinguishing between new cases and chronic cases remains a challenge for HCV surveillance.
Case definitions for surveillance of newly acquired infections remain problematical. The WHO, some European countries, and the USA continue to recommend surveillance of newly acquired symptomatic infection alone despite 85% of infections being asymptomatic. Apart from grossly underestimating the true number of newly acquired cases, the minority of cases that are identified using this approach also fail to represent newly acquired cases in general, as symptomatic cases have been found to differ in their routes of transmission, and natural history [Reference Maheshwari, Ray and Thuluvath12, Reference Haley and Fischer82]. Enhanced surveillance methodologies developed in Canada and Australia that collect information from notifying physicians and laboratories on prior testing histories have been able to identify asymptomatic cases in addition to symptomatic cases. However, these systems rely on the notifying physician providing a HCV testing history for each patient. When cases in an Australian surveillance system were randomly selected for follow-up, a considerable proportion (80%) could not be classified due to lack of available historical testing data. Although the proportion of cases that could be classified as newly acquired increased when cases were targeted for follow-up based on specific criteria, enhanced surveillance continues to be a resource-intensive method for monitoring newly acquired infection [Reference Guy32, Reference Tobin84].
None of the literature reviewed discussed re-infection, so case definitions for newly acquired HCV are likely to have been designed mainly to identify newly acquired primary HCV infection. Indeed, until recently there was little awareness of HCV re-infection [Reference Seeff85]. However, recent studies have shown that re-infection incidence is as high, or potentially higher, than primary infection incidence in PWID [Reference Aitken86–Reference Vickerman94]. While criteria for classifying newly acquired infection based on anti-HCV seroconversion [criterion (d) above] exclude newly acquired re-infection, criteria based on clinical symptoms and/or elevated alanine aminotransferases (ALT) [criteria (b) and (c) above] cannot distinguish between newly acquired primary infection and newly acquired re-infection.
Automated laboratory surveillance has been used in a limited capacity in some jurisdictions and appears to be a promising method for identifying newly acquired cases. Using automated laboratory surveillance, anti-HCV antibody testing history can be collated for individual patients, enabling the identification of newly acquired primary infections [Reference Brant55, Reference Tweed56]. Newly acquired primary infection can be confirmed on the basis of an anti-HCV negative test followed by an anti-HCV positive test or on the basis of a single anti-HCV-negative, HCV RNA-positive test [Reference Hope95–Reference Turner97]. Single anti-HCV-positive tests with elevated ALT results and exclusion of HBV seroconversion may be classified as possible newly acquired cases if there was no previous anti-HCV test; however, in this case it is not possible to classify participants as having primary infection or re-infection.
Although this has not been implemented, if longitudinal HCV RNA testing for individual HCV-exposed patients were available, this would enable automated laboratory surveillance systems to identify newly acquired re-infections in addition to newly acquired primary infections. In this context, the simplest case definition for a newly acquired re-infection is one anti-HCV-positive, HCV RNA-negative test followed by an HCV RNA-positive test. However, this definition lacks specificity because it cannot distinguish between re-infection and reduction in HCV viral load below the limit of detection followed by an increase in HCV viral load. The specificity could be improved by requiring evidence of either multiple consecutive HCV RNA-negative tests prior to the HCV RNA-positive test or a change in HCV genotype. Similar classification schemes for re-infection have been used in longitudinal studies of HCV re-infection in PWID [Reference Aitken86–Reference van de Laar93].
Regardless of the case definitions used, the ability of a linked-laboratory surveillance system to identify newly acquired primary infections, re-infections and/or co-infections will depend on the frequency at which high-risk groups are tested in the community. Currently, there are no Australian, Canadian, or USA HCV guidelines that specify the frequency at which high-risk groups should be tested. The European Monitoring Centre for Drugs and Drug Addiction advise testing PWID for a range of infections including HCV every 6–12 months (although specific laboratory tests are not mentioned) [98]; however, HCV-specific European practice guidelines do not discuss frequency of testing [99–Reference Calvaruso and Craxì102]. If HCV testing frequency guidelines were developed, automated laboratory surveillance systems would provide an opportunity to evaluate implementation through monitoring how often at-risk populations are tested for HCV and which tests are performed.
The surveillance of newly acquired infection has multiple purposes, including some that are motivated at the individual level (e.g. facilitation of early treatment to prevent HCV progression) and some that are motivated at the public health level (e.g. preventing onward transmission of HCV, predicting future disease burden and health system requirements, identifying changes in transmission rates and patterns of infection, detecting outbreaks, and providing the capacity to evaluate the effectiveness of public health interventions). While the ability of the system to deliver on these functions will be sensitive to the HCV testing frequency in the population, it is worth noting that many of these functions could be achieved with testing frequencies of 1–2 years or longer. Studies of combination pegylated interferon-ribavirin treatment for HCV have shown that cases treated within the first 2 years of infection have high treatment success rates [Reference Kamal16–Reference Dore19]; and the introduction of newer, more effective, therapies may mean that this window of opportunity for early treatment will become longer in the future [Reference Chary and Holodniy103, Reference Gane104]. Infections that do not result in spontaneous clearance are responsible for the majority of disease burden, and are also most amenable to treatment interventions, so in order to project future disease burden and health system requirements and facilitate treatment interventions, identifying infections prior to spontaneous clearance is unnecessary. Notably, frequent testing should be targeted to those most at risk of infection and onward transmission. In particular, in order to effectively prevent onward transmission of HCV in PWID, the main consideration is that infections need to be identified while the individual is still injecting, highlighting the importance of regularly testing this group for HCV [Reference Vickerman94].
We acknowledge that the surveillance systems identified are likely to represent only a subset of all HCV surveillance systems operating globally, and that some relevant literature may not have been included due to limiting our review to the English-language literature. Nonetheless, our findings suggests the important field of HCV surveillance has not been sufficiently studied nor communicated, and significant resourcing is required to undertake research into developing surveillance systems that adequately monitor the disease.
Despite the importance of accurately monitoring the extent of HCV transmission in the population through the accurate detection of newly acquired HCV infection, this review found that most HCV surveillance systems are limited in their ability to identify such infections. This finding suggests it is time to rethink how we undertake HCV surveillance. Current case definitions are limited for detecting newly acquired primary infection and do not consider newly acquired re-infection. Passive and enhanced case-ascertainment methods have similarly had limited success in identifying newly acquired infections. Automated extraction of data collected by laboratories is one possible alternative to passive and enhanced surveillance. More research is required to determine whether data-linkage between laboratories can be used to collect longitudinal testing data on individuals who are at risk of acquiring HCV or have already been exposed to HCV, and whether this method can be used to effectively identify new infections, including re-infections. The ability of laboratory surveillance systems to identify newly acquired infections will depend on the testing frequency of at-risk groups; nonetheless, at minimum, automated linked laboratory systems provide an opportunity to investigate and evaluate clinical HCV testing practices.
ACKNOWLEDGEMENTS
We thank Hilary Veale for her assistance reading the literature identified in the database search conducted in March 2011. This work was undertaken as part of a project conducted for the Communicable Diseases Branch Health Protection Directorate Division of the Chief Health Officer, Queensland Health. In addition to Queensland Health, the authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program to the Burnet Institute. Margaret Hellard receives funding from the NHMRC for a Senior Research Fellowship. This work was conducted while Caroline van Gemert was a Master of Applied Epidemiology (MAE) Scholar. The MAE was funded by the Australian Department of Health and Ageing.
DECLARATION OF INTEREST
None.



