INTRODUCTION
Helicobacter pylori is a spiral-shaped, Gram-negative bacterium that establishes chronic colonization in the human stomach and is a causative pathogen of various gastroduodenal diseases, including gastritis, peptic ulcers, gastric cancer, and mucosa-associated lymphoid tissue lymphoma [Reference Peek and Blaser1]. H. pylori infection generally results in chronic gastritis, but a small proportion of infected patients develop more severe diseases such as peptic ulcers and gastric cancer [Reference Fox and Wang2]. In Asia, gastric cancer is a significant health problem with a greatly variable geographical incidence. Based on the age-standardized incidence rate of gastric cancer, Asian countries are categorized as high risk (e.g. Japan, Korea, China), intermediate risk (e.g. Vietnam), or low risk (e.g. Thailand and Indonesia) for gastric cancer [Reference Ferlay3].
Indonesia is a developing country at the southeastern tip of mainland Asia and Oceania; it is an archipelago of more than 13 600 islands with a multi-ethnic society with more than 1000 ethnic and sub-ethnic groups delineated by the Wallace Line, a faunal boundary that separates the ecozones and organisms of Asia and Australia. The age-standardized incidence rate of gastric cancer in Indonesia was reported to be 2·8/100 000, which is relatively low among Asian countries (International Agency for Research on Cancer; GLOBOCAN2012, http://globocan.iarc.fr/). Although the prevalence of H. pylori infections in Indonesia has been investigated, the reports are controversial and contradictory (0–68%) [Reference Tokudome4, Reference Abdullah5]. In addition, to our knowledge, no report has examined H. pylori virulence factors in Indonesian strains. Therefore, it remains unclear whether the low incidence of gastric cancer in Indonesia is due to low infection rates or low H. pylori pathogenicity. In this study, we examined the H. pylori infection rate in a Surabaya hospital using five different tests. We also identified and analysed virulence factors in Indonesian H. pylori strains.
METHODS
Study population
From August 9 to 20 November 2012, 103 consecutive patients with dyspepsia underwent endoscopy at the endoscopic clinic in Dr Soetomo Teaching Hospital, Surabaya, Java island (Fig. 1). Twenty-five patients, including 19 with bleeding related to oesophageal varices and six with history of partial gastric resection, were excluded from this study. Finally, a total of 78 patients with dyspepsia (41 women and 37 men, mean age 49·1 ± 12·4 years, range 14–77 years) were included. The final study population consisted of 43 Javanese, 27 Chinese, four Flores, two Madurese, one Sundanese, and one Batak patient. Experienced endoscopists (U.M. and I.N.) collected four gastric biopsy specimens during each endoscopy session: three samples from the lesser curvature of the antrum about 3 cm from the pyloric ring and one sample from the greater curvature of the corpus. Biopsy specimens for culture were immediately placed under refrigeration at −20°C, and stored at −80°C within a day of collection until used for culture testing. Three antrum specimens were used for H. pylori culture, rapid urease test (CLO test), and histological examination. One corpus specimen was used for histological examination. Peptic ulcers and erosive gastritis were identified by endoscopy. Normal stomach mucosa was defined as the absence of any activity and inflammation in both the antrum and corpus upon histological examination. Patients with evidence of activity or inflammation in the antrum or corpus upon histological examination were considered positive for gastritis. Written informed consent was obtained from all participants, and the study protocol was approved by the Ethics Committee of Dr Soetomo Teaching Hospital (Surabaya, Indonesia) and Oita University Faculty of Medicine (Yufu, Japan).

Fig. 1. Geographical map of Surabaya.
Ethical standards
We declare that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
H. pylori infection status
To maximize diagnostic accuracy, H. pylori infections were diagnosed based on the combined results of five different methods, including culture, histology, immunohistochemistry, rapid urease, and urinary antibody tests. For H. pylori culture, one antrum biopsy specimen was homogenized in saline and inoculated onto Mueller–Hinton II agar medium (Becton Dickinson, USA) supplemented with 7% horse blood without antibiotics. The plates were incubated for up to 10 days at 37°C under microaerophilic conditions (10% O2, 5% CO2, 85% N2). H. pylori bacteria were identified on the basis of colony morphology, Gram staining results, and positive reactions for oxidase, catalase, and urease. Isolated strains were stored at −80°C in Brucella broth (Difco, USA) containing 10% dimethyl sulfoxide and 10% horse serum.
All biopsy materials for histological testing were fixed in 10% buffered formalin and embedded in paraffin. Serial sections were stained with haematoxylin and eosin as well as May–Giemsa stain. Gastric mucosa were evaluated based on the updated Sydney system [Reference Dixon6]. The bacterial load was classified into four grades: 0, ‘normal’; 1, ‘mild’; 2, ‘moderate’; and 3, ‘marked’ [Reference Dixon6]. Samples with bacterial loads greater than or equal to grade 1 were considered positive for H. pylori.
Immunohistochemistry was performed as previously described [Reference Uchida7]. Briefly, after antigen retrieval and inactivation of endogenous peroxidase activity, tissue sections were incubated with α-H. pylori antibody (Dako, Denmark) overnight at 4°C. After washing, the sections were incubated with biotinylated goat anti-rabbit IgG (Nichirei Co., Japan), followed by incubation with an avidin-conjugated horseradish peroxidase solution (Vectastain Elite ABC kit; Vector Laboratories Inc., USA). Peroxidase activity was detected using an H2O2/diaminobenzidine substrate solution.
Urinary H. pylori status was evaluated with a rapid urine test (RAPIRUN® H. pylori antibody, Otsuka Pharmaceutical Co., Japan) according to the manufacturer's instructions. The reported sensitivity, specificity, and accuracy of the kit in the Japanese population have been reported to be 92·0%, 93·1%, and 92·3%, respectively [Reference Yamamoto8]. Immediately after collection, patients' urine samples were tested for H. pylori antibodies. A skilled technician blinded to patients' information measured and analysed all urine samples.
Patients were considered to be negative for H. pylori infection when all five test results were negative, whereas patients with at least one positive test result were considered positive for H. pylori infection.
Determination of gastritis stage
The degree of inflammation, neutrophil activity, atrophy, intestinal metaplasia, and bacterial density were classified into four grades according to the updated Sydney system: 0, ‘normal’; 1, ‘mild’; 2, ‘moderate’; and 3, ‘marked’ [Reference Dixon6]. Samples with grade 1 or more atrophy were considered atrophy-positive [Reference Bornschein9]. In addition, gastritis stage was assessed based on topographic locations (antrum and corpus), according to the Operative Link on Gastritis Assessment (OLGA) system [Reference Rugge10].
H. pylori isolation and genotyping
H. pylori colonies were cultured from antral biopsy specimens using standard methods [Reference Yamaoka11]. H. pylori DNA was extracted from these colonies for H. pylori genotyping using the QIAamp DNA Mini kit (Qiagen, USA) according to the manufacturer's directions. CagA status was determined by polymerase chain reaction (PCR) amplification and direct sequencing of a conserved region of cagA using the previously reported primers cagOMF and cagOMR [Reference Matsunari12]. VacA genotyping (s1 or s2, and m1 or m2) was also performed as described previously [Reference Yamaoka13, Reference Atherton14]. The presence of jhp0562, and β-(1,3)galT were determined based on PCR product size as described previously [Reference Oleastro15]. OipA status (‘on’ or ‘off’) was determined by PCR and sequencing [Reference Yamaoka16]. IceA genotype (iceA1 or iceA2), and dupA prevalence were determined by PCR as described previously [Reference Yamaoka17, Reference Takahashi18]. The amplified fragment was detected by 1·5% agarose gel electrophoresis and ultraviolet transilluminator. DNA sequencing was performed using an AB 3130 Genetic Analyzer (Applied Biosystems, USA) according to the manufacturer's instructions.
Statistical analysis
Data were analysed using SPSS, version 19 (SPSS Inc., USA). Discrete variables were tested using the χ 2 test; continuous variables were tested using Mann–Whitney U and t tests. A two-tailed P value <0·05 was considered statistically significant.
RESULTS
H. pylori infection rate in dyspeptic patients in Surabaya
The total study population of 78 patients with dyspepsia consisted of four patients aged ⩽29 years, 11 patients aged 30–39 years, 30 patients aged 40–49 years, 17 patients aged 50–59 years, and 16 patients aged ⩾60 years. Table 1 shows H. pylori-positive rates for each test. Histology and immunohistochemistry test results were completely concordant. However, rapid urease test results, had the highest positivity rate in this study population (9·0%, 7/78). Three patients were positive for H. pylori by all five tests. Two patients were positive by four tests and negative by the urinary antibody test. Two patients were positive only by the rapid urease test. One patient was positive by histology and another was positive only by the urinary antibody test. When patients were categorized as positive for H. pylori with at least one positive test result, the overall of H. pylori infection rate was 11·5% (9/78). The infection rate by age group was 0% (0/4) for patients aged ⩽29 years, 18·2% (2/11) for patients aged 30–39 years, 10·0% (3/30) for patients aged 40–49 years, 11·8% (2/17) for patients aged 50–59 years, and 12·5% (2/16) for patients aged ⩾60 years. Figure 2 shows the H. pylori infection rate according to age group. There was no statistically significant relationship between H. pylori infection rate and age (P = 0·89).
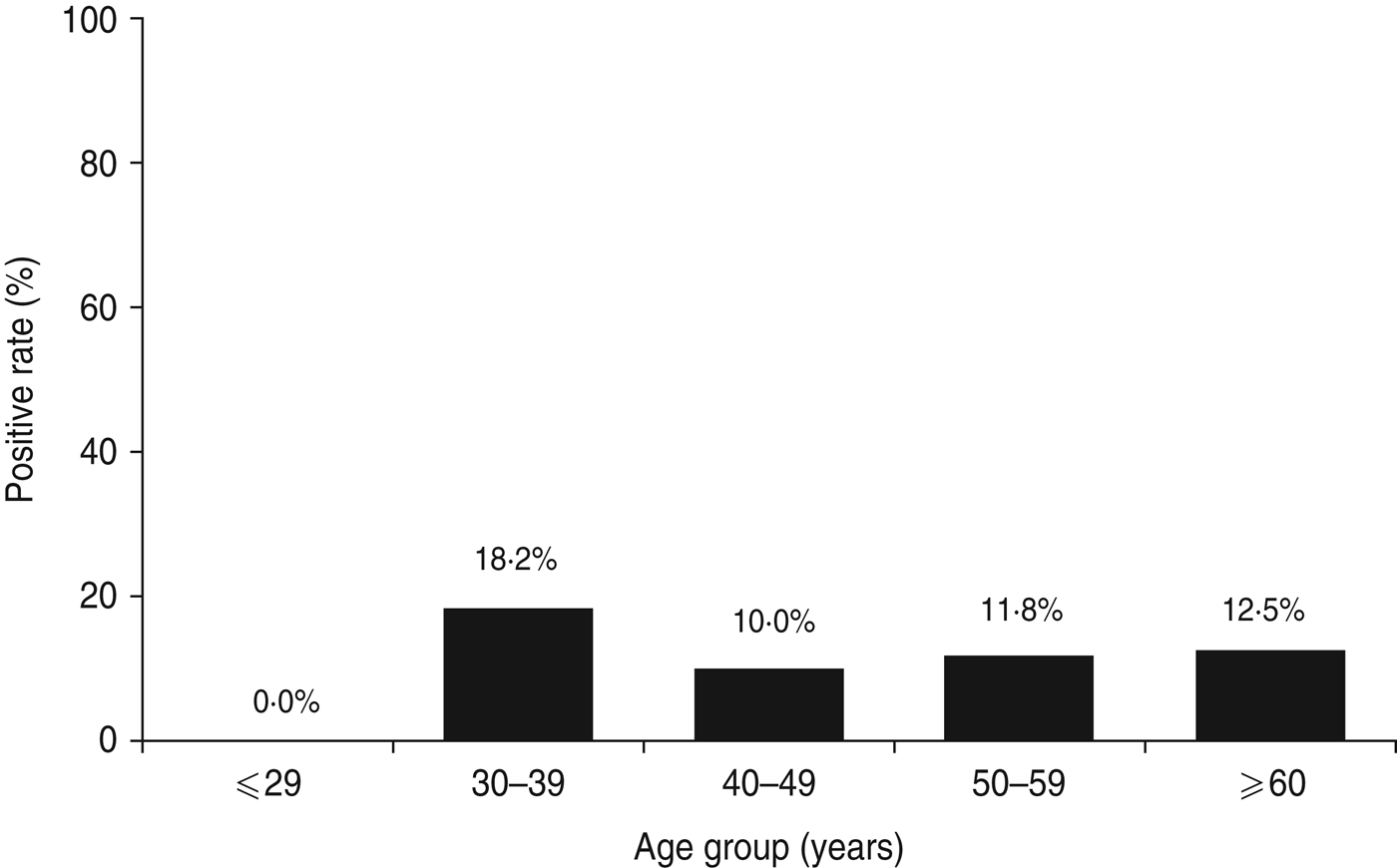
Fig. 2. Helicobacter pylori infection rate in Surabaya by age group. Five different methods were used to test for H. pylori infection, including culture, histology, immunohistochemistry, rapid urease test, and H. pylori urine antibody. Patients were considered negative for H. pylori when all test results were negative; H. pylori-positive status required at least one positive test result.
Table 1. Helicobacter pylori infection rate by diagnostic test
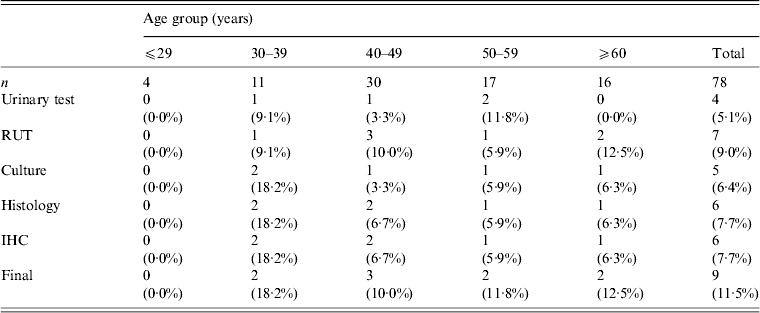
RUT, Rapid urease test; IHC, immunohistochemistry.
H. pylori infection rate according to endoscopic diagnosis
Among 78 patients, 29 showed no activity or inflammation in either the antrum or the corpus by histological examination; these patients were considered the normal group. One of 29 subjects (3·4%) in the normal group was positive for H. pylori infection. However, among 31 patients with gastritis, seven (22·6%) were positive for H. pylori, a significantly higher rate than that in the normal group (P = 0·02). Peptic ulcers were found in eight patients (seven gastric ulcers and one duodenal ulcer). Interestingly, none were infected with H. pylori. The H. pylori infection rate in subjects with erosive gastritis was 10·0% (1/10). No gastric cancer was detected in our study.
H. pylori infection rate according to ethnic group
Among 43 Javanese patients, only one (2·3%) was positive for H. pylori. H. pylori infections were found in 5/27 Chinese (18·0%) patients, a significantly higher rate than that in the Javanese study population (P = 0·01). Two of four Flores patients were positive for H. pylori. One Batak patient was positive for H. pylori infection. Both Madurese and Sundanese patients were negative for H. pylori infection.
Gastric mucosa status
Histological findings showed that 51 patients had grade 0 antrum atrophy; 26 had grade 1, only one had grade 2, and none had grade 3 atrophy. In the corpus, 76 had grade 0 and only two had grade 1 atrophy. Because samples with a grade 1 or more score were considered atrophy-positive, 27 patients (34·6%) had mucosal atrophy in the antrum, and two (2·5%) patients also had corpus mucosal atrophy. Gastritis stage was assessed according to the OLGA system; 65·3% (51/78) had stage 0. Stage I was found in 33·3% (26/78) of patients. One patient (1·2%) had stage II gastritis. Stages III and IV were not found in this study population. Histological scores according to H. pylori infection status are shown in Table 2. The percentage of men tended to be higher in the group positive for H. pylori infection (P = 0·05). However, there were no statistically significant differences in histological scores between men and women (all P > 0·05). Activity in both the antrum and corpus was significantly higher in patients positive for H. pylori than in patients negative for H. pylori [0·56 (1) vs. 0·07 (0) in the antrum, 0·67 (1) vs. 0·06 (0) in the corpus, all P < 0·0001]. In addition, inflammation both in the antrum and corpus was significantly higher in patients positive for H. pylori than in patients negative for H. pylori [0·89 (1) vs. 0·42 (0) in the antrum, 0·56 (0) vs. 0·14 (0) in the corpus, all P = 0·02]. Scores for atrophy in both the antrum and corpus were not significantly different between patients positive and negative for H. pylori infection [0·44 (0) vs. 0·35 (0), P = 0·53 for the antrum, 0·00 (0) vs. 0·03 (0), P = 0·60 for the corpus, respectively]. Overall, only one patient showed moderate gastritis in the antrum. However, she was not infected with H. pylori. Only two patients showed mild atrophy in the corpus; however, they were also negative for H. pylori. No patient positive for H. pylori infection showed corpus atrophy. No patients had intestinal metaplasia irrespective of H. pylori infection. OLGA scores were not statistically different between patients positive and negative for H. pylori infection [0·44 (0) vs. 0·35 (0), P = 0·53].
Table 2. Histological scores according to Helicobacter pylori infection status
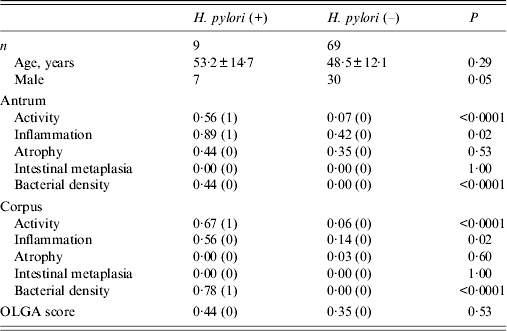
OLGA, Operative Link on Gastritis Assessment.
Age is presented as mean age (±standard deviation), and histology data are presented as mean (median).
H. pylori genotypes in Indonesian strains
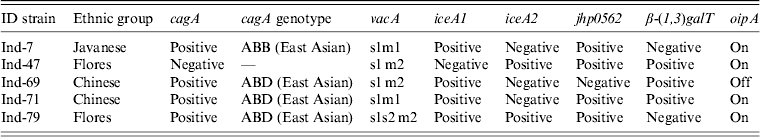
Five strains were successfully isolated and analysed for H. pylori virulence factors. Table 3 shows H. pylori genotypes by ethnic group in these Indonesian strains; cagA was found in four of five strains. Sequence analysis revealed that three strains and one strain possessed East Asian ABD and ABB types, respectively. Figure 3 shows sequence analysis of CagA structural polymorphisms in Indonesian strains. Strains with East Asian-type CagA were isolated from two Chinese patients and one Flores patient. The strain with ABB genotype was isolated from a single Javanese patient. The cagA-negative strain was isolated from a Floresian patient. All cagA-positive strains were oipA ‘on’. VacA analysis showed two s1m1 strains, two s1 m2, and one s1s2 m2 genotypes. IceA1 single positive (iceA2-negative) status was identified in four strains; one strain was positive for both iceA1 and iceA2. One cagA-negative strain was iceA2 single positive. Two strains were jhp0562-positive/β-(1,3)galT-negative. Two strains were double positive for jhp0562 and β-(1,3)galT. One cagA-negative strain had a jhp0562-negative/β-(1,3)galT-positive genotype. Two strains were positive for short-type dupA; no strains were identified with intact long-type dupA genotypes.
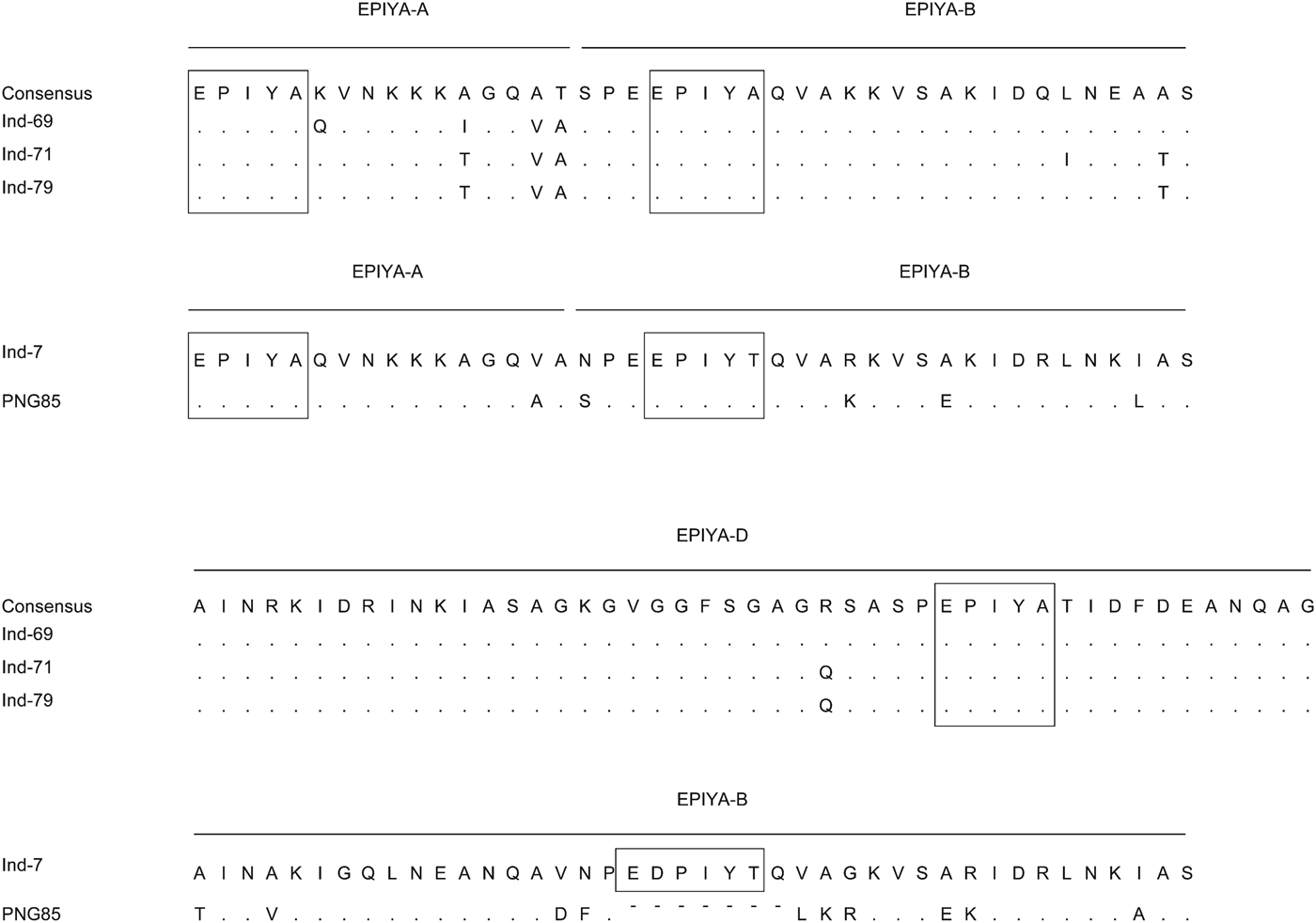
Fig. 3. Sequence analysis of CagA structural polymorphisms in Indonesian strains. Three strains were ABD type. The ABB type was similar to that of strain was classified as hpSahul type by multi-locus sequence typing using seven housekeeping genes.
Table 3. Helicobacter pylori genotypes and patient ethnic groups in strains isolated in Indonesia
Nucleotide sequencing
Nucleotide sequence data for three strains with ABD type and one with ABB type are available under DDBJ accession numbers AB921015 to AB921018.
DISCUSSION
Although we included only patients with dyspepsia in this study, we found a low of H. pylori infection rate in Surabaya, Indonesia, consistent with previous reports [Reference Tokudome4, Reference Tokudome19, Reference Aulia, Manz and Simadibrata20]. In addition, we found severe gastritis and intestinal metaplasia to be rare in in patients from a hospital in Surabaya. This supports suggestions that the low incidence of gastric cancer in Indonesia may be associated with the low H. pylori infection rate and the low prevalence of precancerous legions.
Several studies have examined the prevalence of H. pylori in Indonesia (Table 4). However, the reported prevalence ranged from 0% to 68% [Reference Tokudome4, Reference Abdullah5]. These differences might be attributed to the different study populations and different tests for H. pylori infection. Six studies included patients with dyspepsia [Reference Abdullah5, Reference Aulia, Manz and Simadibrata20–Reference Arinton24], whereas three other studies included study participants from the general population [Reference Tokudome4, Reference Tokudome19, Reference Zhao25]. In nine studies, five used histological examination for diagnosis [Reference Abdullah5, Reference Aulia, Manz and Simadibrata20–Reference Saragih23]. Four studies reported low infection rates (5·7–12·8%) [Reference Aulia, Manz and Simadibrata20–Reference Saragih23]. One study reported a high infection rate (68%) [Reference Abdullah5]; however, these authors did not include their definition for H. pylori-positive status, although they stated that they used a rapid urease test, culture, and histology for diagnosis. The H. pylori infection rate examined by the urea breath test was low in multiple reports (0–11·2%) [Reference Tokudome4, Reference Tokudome19, Reference Zhao25]. One study used PCR methods to detect H. pylori ureC [Reference Arinton24] and found a high H. pylori-positive rate (41·9%).
Table 4. Summary of previous Helicobacter pylori prevalence studies in Indonesia

UBT, Urea breath test; PCR, polymerase chain reaction.
* This study tested for H. pylori by histology, culture, and rapid urease test.
Therefore, it is necessary to recognize differences in H. pylori test accuracy. For example, 54 (85·7%) of 63 dyspeptic patients were positive based on rapid urease testing and microscopic detection of H. pylori [Reference Syam21]. However, among these patients, 42 were positive by only stool antigen test, which suggests the potential for false-positive results. In addition, differences in the results by histological examination might be due to the different evaluation criteria adopted by different studies. This could be overcome by the standardization of biopsy location and instrument, sample size, and using the same pathologists to read results. Different kit types may also contribute to different results. Tokudome et al. examined patients' serum for H. pylori antibodies using an enzyme-linked immunoassay (ELISA) kit (Kyowa Medex Co., Japan) produced and tested in Japan [Reference Tokudome19]. Unfortunately, the authors did not mention the accuracy of the ELISA test kit was determined using antigens extracted from Japanese strains. It is important to develop ELISA kits using H. pylori strains native to the study population.
In the present study, we used five different H. pylori tests to increase diagnostic accuracy as well as to compare results among tests. We found that the H. pylori infection rate was very low, irrespective of the test. We previously reported the prevalence of H. pylori infection in Bhutan using the same criteria [Reference Vilaichone26], although we substituted urinary testing for serological testing in this study. The H. pylori prevalence was quite high (73·4%) in Bhutan; the concordance between different tests was also very high [Reference Vilaichone26]. Importantly, the same pathologist (T.U.) and microbiologist (M.M.) performed the experiments in both studies, which suggests a very small potential for bias. Furthermore, our preliminary study showed complete concordance between serology and urinary test results in Manado, Indonesia (M. Miftahussurur and Y. Yamaoka, unpublished data). These results suggest that our H. pylori infection criteria are reliable. Even when patients with a single positive test result were considered positive for H. pylori infection, the H. pylori infection rate in our patients from a Surabaya hospital was only 11·5% (9/78). The rapid urease test showed the highest positive rate (9·0%). Among nine patients, only three were positive by all five tests. Our data confirmed that the H. pylori infection prevalence is quite low in patients from a Surabaya hospital. In our study, severe gastritis and intestinal metaplasia were also rare in Indonesia. Consistent with this observation, Abdullah et al. found that the grade and activity of gastritis and mucosal atrophy was higher in Japanese than in Indonesian patients positive for H. pylori [Reference Abdullah5]. That difference may explain the disparity in the incidence of gastric cancer between Indonesia and Japan. However, activity and inflammation in both the antrum and corpus were significantly higher in patients positive for H. pylori than in negative patients in Indonesia. Furthermore, although no patient had intestinal metaplasia irrespective of H. pylori infection in this study, other research in the Malay ethnic group has found intestinal metaplasia and dysplasia to be significantly associated with H. pylori infection even in regions with low H. pylori prevalence [Reference Yeh27]. These results suggest that H. pylori should be eradicated even in areas with low H. pylori prevalence.
The low H. pylori infection rate in Indonesia is a different trend compared to other developing countries. In general, environmental factors, such as poor living conditions, are associated with higher H. pylori infection rates. However, sanitary conditions (food hygiene and drinking water) alone cannot explain the low H. pylori infection prevalence in Indonesia, because approximately 50% of the population in Indonesia still use basic environmental conditions for sanitation (UNICEF, http://www.unicef.org/). A low H. pylori infection rate was also reported in the neighbouring country of Malaysia. Similarly, the incidence of gastric cancer is also low in Malaysia. Host genetic factors might contribute to a reduced susceptibility to H. pylori infection, a possibility suggested in the ethnic Malaysian population [Reference Maran28, Reference Lee, Mahendra Raj and Graham29]. Other environmental factors such as the frequent use of ‘budu’ or local anchovy sauce, and ‘pegaga’ or centenella asiatica have also been reported to be associated with the low prevalence of H. pylori in Malaysia [Reference Lee30]. Further studies of host and environmental factors in Indonesia are necessary to better elucidate reasons for the low H. pylori infection prevalence in Indonesia and Malaysia.
Previous studies used H. pylori strains isolated from Javanese patients. Although the number of subjects was small, to our knowledge, this is the first study to compare the H. pylori infection rate in different ethnic groups. Interestingly, the highest H. pylori rate was found in patients from the Chinese Indonesian population instead of patients from the Javanese population. However, the prevalence of H. pylori infection in Indonesians of Chinese descent was lower than that of Chinese non-immigrants [Reference Shi31]. Environmental factors might contribute to the lower H. pylori infection rate in Chinese Indonesians. The transmission routes of H. pylori are still not entirely understood, but human-to-human spread through oral–oral or faecal–oral routes are considered the most plausible routes for infection [Reference Goh32]. Therefore, intra-racial or intra-community spread such as transmission from mother to child might contribute to these racial differences in H. pylori infection rates. Although only one isolate was isolated from a Javanese patient, it had an ABB type. Interestingly, sequence analysis showed that the cagA repeat region of this strain was similar (homology 90·5%) to that of strain PNGhigh85 (Fig. 3), which was isolated in Papua (New Guinea) and classified as hpSahul type by multi-locus sequence typing using seven housekeeping genes [Reference Olbermann33]. The eastern sections of Indonesia, especially Papua, were geographically connected to Australia as a single continent (Sahul) about 60 000 years ago; the Javanese isolate might have some historical connection with the Sahul-type strain. A larger sample size is necessary to elucidate the origin of H. pylori strains in Indonesia.
Although the number of samples was not sufficient for statistically significant conclusions, we also examined H. pylori virulence factors in Indonesian strains in detail. In general, cagA positive (especially East Asian-type cagA), vacA s1m1, oipA ‘on’, iceA1 positive, jhp0562-positive/β-(1,3)galT-negative, and intact long-type dupA positive are considered to be more virulent [Reference Shiota, Suzuki and Yamaoka34]. Our study revealed that some strains had this more virulent genotype. Further studies with increased sample numbers are necessary to better elucidate the virulence of Indonesian H. pylori strains. Furthermore, an increased number of samples might be useful to clarify the association between H. pylori genotype and ethnic groups in Indonesia.
Our study has several limitations. First, we could not obtain information about medications used by the study participants. Therefore, it is possible that we included patients who had been administered antibiotics, histamine-2 receptor antagonists (H2 blockers), or proton pump inhibitors, which can influence H. pylori infection prevalence. However, a previous report found that the prevalence of H. pylori infection in Indonesia was quite low (10·2%) even when patients taking proton pump inhibitors were excluded from the study population [Reference Syam22]. Interestingly, none of the eight patients diagnosed with peptic ulcers were positive for H. pylori in our study. A recent report in an elderly population found that the absence of H. pylori infection did not reduce the risk of bleeding peptic ulcers in patients with other risk factors, especially those who were receiving drug treatments [Reference Lee35]. Unfortunately, we did not obtain information on the usage of non-steroidal anti-inflammatory drugs that are also an important factor for the development of peptic ulcers [Reference Malfertheiner36]. Further information is necessary to elucidate the mechanisms of peptic ulcer development in Indonesia. Second, we obtained samples from a hospital in Surabaya, which located in the eastern part Java island and the second largest city in Indonesia. Sanitary conditions vary by area in Indonesia, although they are generally better in western regions than in eastern areas. Therefore, our results cannot be generalized across Java or Indonesia. In addition, we included only patients with dyspepsia in our study population, and not members of the general population. In Indonesia, many patients with dyspepsia are not covered by the Indonesian health insurance system. Therefore, it is difficult for them to undergo endoscopy. Further investigation from all regions of Indonesia is necessary to elucidate the reasons for the low rate of gastric cancer.
In conclusion, we found a low of H. pylori infection rate in dyspeptic patients in Surabaya. In addition, we found severe gastritis and intestinal metaplasia to be rare even in patients positive for H. pylori infection. Our findings support previous reports of low incidence rate of gastric cancer in Indonesia and may be attributed to the low H. pylori infection rate and low prevalence of precancerous legions. However, activity and inflammation in both the antrum and corpus were significantly higher in patients with H. pylori than that in those without. Some H. pylori strains were of more virulent genotypes. Therefore, early diagnosis and treatment of H. pylori infection is necessary for symptomatic patients in Surabaya to reduce chronic complications risk.
ACKNOWLEDGEMENTS
This report is based on work supported in part by grants from the National Institutes of Health (DK62813) (Y.Y.), and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (22390085, 22659087, 24406015 and 24659200) (Y.Y.), (23790798) (S.S.). This work was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency (JST).
DECLARATION OF INTEREST
None.










