INTRODUCTION
Brucella canis is a Gram-negative, aerobic, intracellular coccobacillus first recognized in 1966 [Reference Carmichael1], isolated from tissue and vaginal discharges of infected dogs. Cultures are always in the rough or mucoid phase on primary isolation. The Brucella Subcommittee meeting in Munich in 1978 [Reference Osterman and Moriyon2] recommended that it be given full species status.
Outbreaks of canine abortions caused by B. canis have been widely reported [Reference Carmichael, Nielsen and Duncan3–Reference Carmichael and Shin5] describing clinical signs ranging from asymptomatic to abortion and testicular atrophy [Reference Hollett6].
The zoonotic risk is relatively high in persons who handle breeding dogs in kennels and are exposed to reproductive tissues and fluids of infected dogs [Reference Hollett6]. Transmission to humans in other circumstances has been considered rare [Reference Wanke7] with only 30 cases reported worldwide since the first isolation in the late 1960s [Reference Hollett6]. Other authors consider B. melitensis and B. suis more virulent for humans than B. abortus and B. canis [Reference Young8].
In this paper we present results from an uncommon outbreak of human brucellosis, which to the best of our knowledge is the first reported in the literature to be caused by B. canis.
MATERIALS AND METHODS
Index case
A 17-month-old boy was admitted to the Paediatrics Department of the Eva Perón Hospital of San Martín, Buenos Aires with a 72 h history of watery diarrhoea, watery vomiting, fever (39°C) and dehydration 5%. Gastroenteritis was suspected because of vomiting without diarrhoea 1 week earlier which had resolved spontaneously. Co-proculture was negative and CSF by lumbar puncture was normal, no organisms growing in culture of this sample. Laboratory tests showed leukocytes 7·3×109/l, lymphocytes 29·4%, monocytes 9·6% and granulocytes 61%. Red cell count was 5×109/l, haemoglobin 12·3, haematocrit 36%, platelets 2·77×105, creatinine 0·30, urea 0·12 and glycaemia 0·9. Iontophoresis: potassium 3·4, sodium 130 and chloride 101.
The patient received oral glucose electrolyte solution therapy and 100 mg/kg ceftriaxone twice a day. After 3 days of antibiotic treatment he showed remarkable clinical improvement, antibiotic therapy was suspended and the patient released from the hospital. Three days afterwards the two blood cultures (Bact Alert, bioMérieux, France) were positive and a small non-mobile Gram-negative coccobacillus was isolated. The strain grew without producing acid on triple sugar iron (TSI) agar and was positive to urease, nitrate reduction and oxidase tests. It was tentatively identified as B. canis and sent to our laboratory for confirmation. Conventional brucellosis serological tests had not been performed on the patient's serum.
Epidemiological investigation
Because of suspicion of canine brucellosis the family of the index case was traced. They had two dogs, a male and a female; the latter had recently had five puppies, two stillborn. The dogs and three puppies were clinically examined and tested for brucellosis. The family had kept one female puppy and given the two males to two other families. The index case family (10 members), six members of the family in contact with one male puppy and seven of the family with the second puppy were tested for brucellosis.
Serological tests
For detection of smooth Brucella antibodies: we ran the buffered plate agglutination test (BPAT), Rose Bengal test (RBT), standard tube agglutination test (STAT) and complement fixation test (CFT) using antigens prepared at ANLIS by Dr C. G. Malbrán with B. abortus 1119-3 strain. Competitive ELISA (CELISA) were performed as previously reported [Reference Lucero9] with antigen (S-LPS from B. abortus 1119-3) and the mAb standardized and supplied by the Brucellosis Centre of Expertise and OIE Reference Laboratory, Animal Diseases Research Institute (ADRI), Canada. The test is positive when %I>28.
For detection of rough Brucella antibodies we used rapid screening agglutination test (RSAT) to detect anti-B. canis antibodies, including a control standard serum with each test. This antigen was prepared at ANLIS by Dr C. G. Malbrán from the (M-) variant strain of B. canis.
Indirect ELISA (IELISA) with B. canis antigen was used as a confirmatory test for the detection of human [Reference Lucero10] and dog [Reference Lucero11] anti-B. canis antibodies including positive, weak positive and negative sera as controls in each plate. In order to detect human anti-B. canis antibodies we used a previously established cut-off value of %P>27; for dogs the value was %P>29. A recombinant protein combining immunoglobulin-binding sites of proteins A and G conjugated with horseradish peroxidase was used for assessment of antibodies to rough lipopolysaccharide in dogs and humans [Reference Lucero10].
Clinical isolates
For dog blood cultures we used monophasic commercial liquid medium Hemo Brucella (Britania SA, Argentina).
Bacteriological studies
The strains isolated were identified and typed by CO2 requirement and its agglutination pattern with monospecific anti-A, anti-M and anti-R sera. Brucella cultures are smooth or rough, agglutinated by their respective antisera. Smooth-form cultures may be examined for their predominant agglutinogen A (B. abortus, B. suis) or M (B. melitensis) but rough-form cultures are agglutinated by unabsorbed antisera prepared with B. canis or B. ovis cultures. Urease test, production of H2S, growth on dyes, erythritol and penicillin sensitivity and lysis by Tb, Wb and R/C phages were performed following procedures previously described and included typed Brucella strains of each species in all tests [Reference Corbel, Banai, Brenner, Krieg, Staley and Garrity12, Reference Alton13]. Colony morphology was studied initially by direct observation, acriflavine test and staining of colonies with Crystal Violet.
PCR of strains isolated
A previously described [Reference Imaoka14] combinatorial PCR was performed. DNA of strains was amplified using puReTaq Ready-To-Go PCR Beads (GE Healthcare Bio-Science Corp., USA). We were able to identify B. canis when the primer BCSP31 and omp31 were amplified and amplicons of omp2b and omp2a were detected by B. canis-specific primers but not by B. abortus-specific primers.
RESULTS
Serological tests using B. abortus 1119-3 antigen were negative for the patients, but when B. canis antigen was used, three members of the index case family, two of the second family and one of the third family tested positive with titres declining over time (Table 1). The bitch and three puppies also tested positive (Table 1).
Table 1. Serology, bacteriology and clinical findings in human cases and dogs
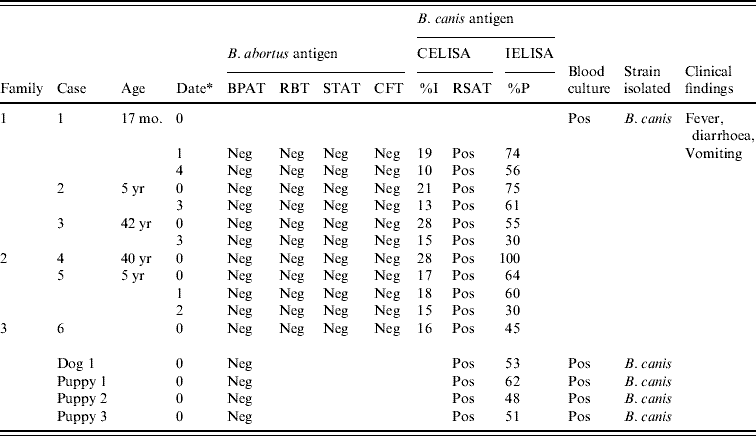
CELISA, Competitive ELISA; IELISA, indirect ELISA; BPAT, Buffered plate agglutination test; CFT, complement fixation test; Neg, negative; Pos, positive; RBT, Rose Bengal test; RSAT, rapid slide agglutination test; STAT, standard tube agglutination test.
CELISA cut-off (I%) >28; Dogs IELISA cut-off (%P)>29; Human IELISA cut-off (%P) >27.
* Date=months after first consultation.
Five strains were isolated: one from the index case and four from dogs in contact with him. Conventional biochemical tests performed on strains were consistent with B. canis (Table 2); subsequently, combinatorial PCR confirmed these results.
Table 2. Differential characteristics of strains isolated and species of genus Brucella
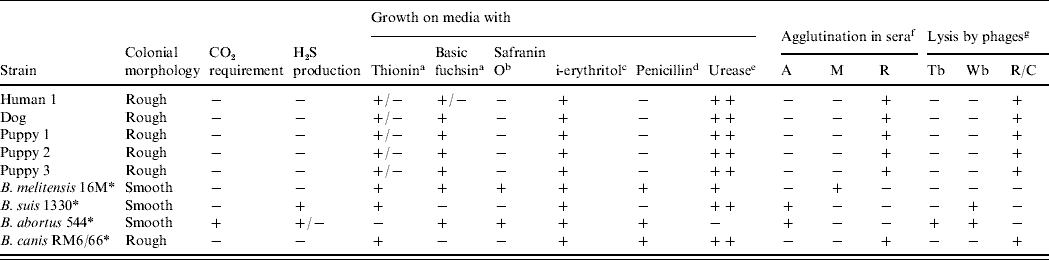
a 20 μg/ml; b 100 μg/ml; c 1 mg/ml; d 5 IU/ml in base medium; e Bauer's method; f A, A monospecific antiserum; M, M monospecific antiserum; R, rough Brucella antiserum; g at routine test dilution (RTD).
* Reference strains.
After confirmation of strain as B. canis the index case was cited and placed on 5 mg/kg trimethoprim–sulfamethoxazole/20 mg/kg rifampicin daily for 6 weeks. Seventy-two hours later he presented mild fever and nasal congestion probably due to an associated viral infection. At the end of antibiotic therapy he was much better. His 5-year-old brother with positive RSAT and IELISA was examined clinically showing palpable spleen and 19200 wbC. He suffered from an episode of vomiting lasting for 3 days one month previously and at the time of the examination presented asthenia and loss of appetite. He was placed on 5 mg/K trimethoprim–sulfamethoxazole/20 mg/kg rifampicin daily for 40 days. Two months later he had recovered. The mother with positive RSAT and IELISA reported asthenia as her only symptom and after clinical examination no treatment was indicated. Neither signs nor symptoms of relapse were detected during the follow-up period (6 months) in the outpatient service where index case, his brother and mother were evaluated.
The mother of the second family which had been in contact with puppies reported headache, asthenia, myalgias and nausea over the last 2 months but refused clinical examination. Her 5-year-old daughter had suffered from fever of unknown origin, vomiting and diarrhoea 2 months previously. The girl had a high (>64) c-reactive protein and was placed on 5 mg/kg trimethoprim–sulfamethoxazole/20 mg/kg rifampicin daily for 40 days but therapy was abandoned after 3 weeks. One member of the third family that received a puppy was positive to RSAT and IELISA, asymptomatic and after clinical examination no therapy was indicated.
DISCUSSION
No clinical signs are pathognomonic of canine brucellosis, although reproductive failure and infertility should be suspected. Transmission is mainly through contact with vaginal discharges, abortion materials and fluids of bitches and semen and/or urine of males [Reference Carmichael, Nielsen and Duncan3]. Since clinical examinations are inadequate for diagnosis, isolation of the organism and serological tests are the only reliable way to confirm a presumptive diagnosis. In this case the bitch had a history of abortion 3 years previously, gave birth to weak puppies which died after 3 days one year later, but was never diagnosed with brucellosis. Of the last pregnancy in 2008, two puppies were born dead and three (two males, one female) were apparently normal.
This situation, associated with a recent study of 219 dogs in lower-class neighbourhoods and slums of Buenos Aires with a high rate of unmet basic needs, which found anti-B. canis antibodies in 7·3% of dogs and B. canis isolations in three cases, indicates a health hazard for the population exposed [Reference Boeri15]. More recently 224 dogs tested for canine brucellosis in the context of a free neuter programme in another area of Buenos Aires found 10·7% serologically positive dogs while B. canis was isolated in two cases [Reference López16]. Since infected dogs have been shown to remain bacteraemic for long periods of time, these results also suggest a risk of human infection in this area.
Considering the few reports of human global cases in the past 20 years, B. canis is probably either not tested for or not reported [Reference Carmichael and Shin5]. Since routine human brucellosis diagnosis does not include B. canis investigation, infection with this species may be more widespread than is currently suspected [Reference Lucero10].
This outbreak involved six persons (three children, three adults) and four dogs living in close contact with the family. The clinical symptoms of the index case led to an erroneous diagnosis and the infection would have remained undiagnosed if culture had not been positive. Human brucellosis is usually described as a disease with protean manifestations and should be suspected, especially in endemic areas. Awareness of canine brucellosis in humans is low, including knowledge of its transmission potential and its medical consequences. Identification of a human case should prompt investigation in order to enable early detection and treatment of all patients. This report aims to increase the awareness of medical personnel of the need to order screening tests for children, immunodeficient persons or pregnant women presenting fever of unknown origin, unexplained spleen or liver enlargement or other systemic signs.
Because this study was based on clinical observations of this case alone, we were unable to evaluate the contribution B. canis to other possible cases in the same neighbourhood. All ages were affected, probably because of close contact with the bitch and puppies; however, children, particularly those aged <6 years might be more exposed because they play with dogs more often and are less protected. The family may have become infected by the latest whelping because they were in contact with the puppies whereas the two previous episodes were miscarriages.
After the outbreak, all dogs were removed from the house to the Anthropozoonosis Centre where one puppy died. B. canis was isolated from spleen, axillary lymph nodes, thymus, pleurae and liver after autopsy. No strain was isolated from mediastinal lymph nodes. The bitch and two surviving puppies were neutered, placed on antibiotic therapy and checked periodically by serological and bacteriological tests.
Control measures including examination of dogs in the neighbourhood and a campaign for information and education of the community were developed by the Anthropozoonosis Centre. The emergence of this urban outbreak also demonstrates the importance of coordinating canine brucellosis surveillance systems.
ACKNOWLEDGEMENTS
We are very grateful to Dr S. Cravero of the Instituto de Biotecnología, CICVyA, INTA, Castelar, Buenos Aires, Argentina, for combinatorial PCR.
DECLARATION OF INTEREST
None.




