INTRODUCTION
Trichinellosis is a zoonotic disease that is caused by nematode parasites of the genus Trichinella. The life-cycle can take place in many different, carnivorous and omnivorous animal species, including humans, by infection after oral ingestion of infective larvae present in striated muscles of infected animals. In the epithelial cells of the small intestines infective larvae develop to adult male and female parasites and after mating, newborn larvae migrate via blood and lymphatic vessels to the favoured sites and finally encapsulate in striated muscle cells ready to infect the next host.
In Europe, the most important sources for human infection are improperly processed infected meat of domestic pig, horses and wild boars. Since trichinellosis is considered a serious disease in humans, meat inspection (based on the testing of an appropriate amount of meat sampled after slaughtering) is a historical keystone in European policy for preventing clinical symptoms in humans. This is particularly relevant for international trade of meat and meat products because of regional differences in the prevalence of this parasite in Europe. In the European Union (EU) all meats marketed for human consumption must be inspected for larval Trichinella [1]. Testing is based on risk assessment to prevent human clinical illness by the assumption that the minimal infectious dose for humans is between 60 and 750 infective larvae [1] and thus testing should indicate the absence of Trichinella in 1 g pig meat and 5 g horse or wild boar meat, considering an average human portion of 100 g meat. Clinical illness in humans is dependent on the dose ingested, and can range from asymptomatic to severe illness and mortality; however, scientific evidence of the dose response in humans is still lacking.
The first step in estimating the health risks associated with Trichinella in meat and meat products is assessment of human exposure to living parasites, viable for infection. When the dose is known, health effects resulting from exposure must be quantified. A dose–response relationship quantitatively describes the probability of infection and/or illness as a function of exposure. Quantitative risk assessment should incorporate an empirical assessment of human susceptibility. When the dose–response relationship is known any exposure (estimated number of larvae ingested) can be translated into a probability that this exposure causes infection. Conversely, any arbitrary level of risk can be translated into a corresponding level of exposure, aiding the setting of standards, e.g. by regulating agencies.
For several microbial pathogens dose–response assessment has been based on human challenge studies, in which human volunteers have ingested defined doses of pathogens. This is only ethically acceptable for mild pathogens that do not cause much discomfort, and certainly do not inflict much damage on the volunteers. The seriousness of the symptoms caused by Trichinella makes it unlikely that volunteer studies will be done in humans.
There are, however, many reported outbreaks of trichinellosis in humans. In some of these outbreaks a sample of the contaminated food was available for analysis, and some of these samples did produce a useful count of the number of larvae. Such occurrences may be used to assess the dose–response relationship [Reference Teunis, Takumi and Shinagawa2, Reference Teunis, Ogden and Strachan3].
A literature study, combined with a questionnaire to participants of the Trichimed network, produced a set of usable outbreaks, shown below. We show that these outbreak reports allow estimation of the infectivity of Trichinella in humans, in turn making human risk assessment possible.
MATERIALS AND METHODS
Data from Trichinella outbreaks
In order to be used for dose–response assessment, outbreak reports must at least provide data on numbers of subjects exposed to the contaminated food, and numbers of subjects who developed symptoms. Further, information allowing estimation of the ingested dose must be present: concentration of larvae in the contaminated food and the amount of food that was consumed by exposed persons.
Preferably, these data of exposure should allow assessment of heterogeneity in exposure. Some subjects may have eaten more than others, and some parts of the infected animal may have contained more larvae than other parts. Information of such factors determining heterogeneity in exposure increases the usefulness of the data.
Fortunately, suspected cases of trichinellosis are often tested serologically, and in a cluster of cases asymptomatic subjects who also consumed infected meat are tested as well. In principle, this allows detection of asymptomatic infections: serologically positive (seroconverting) individuals without symptoms. In most of the incidents used in the present study, all infected subjects became ill, and we concluded that human exposure nearly always results in illness.
Considerable numbers of outbreaks or incidents of trichinellosis are reported, but only a small proportion of these produced the necessary information for quantitative study of infectivity. We succeeded in collecting data on nine outbreaks over a period of 7 years (2000–2006).
Dose–response model
Muscles can become infected with larvae if and only if ⩾1 female and male pathogens survive. If female and male pathogens have equal survival probabilities p m, and female and male pathogens are present in proportions r and 1− r (r is the sex ratio; i.e. the fraction female larvae), then the probability of infection is
assuming Poisson exposure with dose CV: intake of volume V, with concentration C, pathogens per unit volume (or mass).
In case of a sexual reproduction cycle, the dose–response relationship can thus be expressed as a linear combination of three terms, each equivalent to the simple exponential dose–response model for asexual pathogens [Reference Teunis and Havelaar4].
For heterogeneous (beta-distributed) p m the dose–response relationship can be written as a linear combination, of confluent hypergeometric functions [Reference Teunis, Takumi and Shinagawa2]
![\eqalign{ P_{{\rm inf}} \lpar C \cdot V\vert \alpha \comma \beta \comma r\rpar \equals \tab 1 \plus _{\setnum{1}} F_{\setnum{1}} \left[ {\alpha \comma \alpha \plus \beta \semi \minus C \cdot V} \right] \cr \tab \minus _{\setnum{1}} F_{\setnum{1}} \left[ {\alpha \comma \alpha \plus \beta \semi \minus C \cdot V\lpar 1 \minus r\rpar } \right] \cr \tab \minus _{\setnum{1}} F_{\setnum{1}} \left[ {\alpha \comma \alpha \plus \beta \semi \minus C \cdot Vr} \right] .\cr}](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20160921000812316-0649:S0950268811000380:S0950268811000380_eqn2.gif?pub-status=live)
In case the dose also has extra-Poisson variation, with dispersion factor ρ [Reference Teunis, Ogden and Strachan3], the resulting dose–response relationship is a linear combination of another hypergeometric function
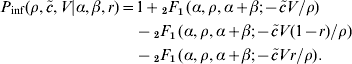
The influence of the sex ratio r on the shape of the dose–response relationship is strongest when its value is close to 0·5. Such values of r tend to steepen the slope of the dose–response relationship. For extreme values (either near 0 or 1) there is a shortage of females or males, which then becomes the limiting factor in infection and acts in downscaling the dose (shifting the dose–response relationship to the right on the dose axis, without changing its shape).
Statistical methods
The likelihood is binomial: for each incident K out of N subjects exposed to a dose D=g(ρ, ![]() , V) have been observed to be affected.
, V) have been observed to be affected.
Given the hit theory dose–response relationship, a single observed fraction infected (i.e. response) may allow prediction of the dose–response relationship [Reference Teunis, Takumi and Shinagawa2]. We wished to incorporate data from several outbreaks with different levels of exposure, and a single response rate at each dose. However, such an approach inevitably involves an additional level of biological variation. While a different human population, similar in age and health status might have similar susceptibility, a different isolate of the pathogen is likely to have completely different infectivity, if only because of a different history (different food vehicle, different previous host). Therefore, analysis of data from different outbreaks requires a hierarchical model.
The dose is characterized by the expected concentration of pathogens, and their dispersion, characterized by the gamma shape parameter ρ [Reference Teunis, Ogden and Strachan3]. These two parameters (dose and dispersion parameter) were estimated separately using whatever information was available in the outbreak reports. In most outbreak reports a range was provided for the intake of contaminated unheated (or inadequately heated) meat.
RESULTS
Outbreak data
For all used outbreaks, a number of Trichinella larvae/g contaminated meat was reported. Moreover, some information on the intake of contaminated meat was available, varying from a direct indication of the consumed amount to information on the type of meal in which the meat was used.
Table 1 lists this information for the admissible outbreak reports. Additional details are given below:
Ranque et al. 2000 [Reference Ranque5]: Four human cases exposed to T. pseudospiralis in France, two patients ate <300 g, the other two ate >300 g wild boar meat with an estimated 187 larvae/g.
Pozio et al. 2006 [Reference Pozio6]: Eleven people ate raw sausages containing 8 larvae/g T. britovi, 10 people were symptomatic, one asymptomatic.
Gari-Toussaint et al. 2005 [Reference Gari-Toussaint7]: Six people consumed frozen wild boar meat with 3 larvae/g T. britovi, all were infected (all symptomatic).
Turk et al. 2006 [Reference Turk8]: At a wedding 474 people ate raw meatballs with 6·5 larvae/g T. britovi. Of these, 154 were confirmed with trichinellosis, of the remaining exposed individuals, 71 were initially diagnosed with highly likely trichinellosis, 60 with probable trichinellosis, 42 with suspected trichinellosis, and 147 with highly unlikely trichinellosis. All these latter subjects appeared to be seronegative.
Ancelle et al. 2006 [Reference Ancelle9]: A bear shot in Canada appeared to be highly infectious with 295 larvae/g T. nativa, resulting in three clusters of cases. Of the 10 hunters who all consumed meat, eight developed clinical disease. In Orleans six people ate the same meat, five of whom became ill. Further, 2 weeks later in Narbonne, nine people ate the same meat, and four of these became infected and ill. At the time of the last cluster, the first patients had been identified and treated. The cases in Narbonne were also treated at the same time, shortly after they had been exposed. Therefore this latter cluster may show lower infectivity, as some of the exposed subjects may have been saved from developing infection.
Littman et al. 2006 [Reference Littman, Nöckler and Hallauer10]: Consumption of meat and meat products from a home-reared pig containing approximately 106 larvae/g T. spiralis by 22 people resulted in 18 infected cases, 17 of whom were symptomatic.
Rouen 2004 [Supplementary Table A1 (available online)]: Black bear meat infected with T. nativa produced one case who consumed 300 g containing 250 larvae/g.
Nans les pins 2006 (Supplementary Table A1): Three cases caused by consumption of wild boar meat with about 40 larvae/g T. spiralis.
Collobriëres (Supplementary Table A1): Ten people consumed wild boar meat with 5–10 larvae/g T. britovi, four were symptomatic.
Table 2 shows exposure parameters and response data for the nine outbreaks included.
Table 1. Data used for exposure assessment. The concentration of larvae in the implicated meat has been determined from stored samples. To quantify the distribution of the amounts of contaminated meat consumed lower and upper limits have been determined as percentiles

Table 2. Outbreak data used for dose–response assessment. Exposure characterized by larvae concentration in contaminated meat, average intake and extra-Poisson dispersion parameter (ρ). Infection characterized by elevated levels of antibodies against Trichinella

* Narbonne cluster, not included.
Studies of the sex ratio of trichinellae in the intestines are rare; Christenson [Reference Christenson11] fed rats with meat containing high numbers of larvae of T. spiralis and initially (after challenge) found equal numbers of female and male worms. A few days later the fraction of males started to decrease, as did the total numbers of worms in intestinal contents. Gursch [Reference Gursch12] also observed decreasing numbers of intestinal worms as infection (T. spiralis larvae migrating into muscle tissue) progressed, but found that initially females outnumbered males by a factor of about 2:1, while later (10–14 days) the sex ratio decreased. In a study in hamsters, Boyd et al. [Reference Boyd and Huston13] found similar results: initial ratios close to 2:1, later (6 days) decreasing to 1·5:1. In a more recent study Leiby & Bacha [Reference Leiby and Bacha14] found similar sex ratios in mice infected with T. spiralis larvae from three different sources. Therefore we used a fraction of 0·7 females (and 0·3 males), corresponding to a sex ratio of about 2:1 in the present study.
Dose–response relationships
Figure 1 shows fitted dose–response relationships [equation (3)] for each of the included outbreaks. The variation in location of the curves illustrates possible biological variation in infectivity of larvae from different sources, in different host populations, and in different food media. Also clearly visible in this graph is the difference in slopes of the curves, indicating heterogeneity from a combination of pathogen factors (differences in within outbreak variation in infectivity) and inoculum factors (differences in extra-Poisson variation in pathogen occurrence). The Narbonne cluster [Reference Ancelle9], where the attack rate is substantially lower than in the two remaining clusters from the same outbreak, has been omitted.

Fig. 1. Outbreak-based Trichinella dose–response for infection: individual best (posterior mode) relationships for each of the 10 data-points from nine outbreaks (nine curves, as Ranque et al. [Reference Ranque5] contributes two different doses). Numbers indicate species: 1, spiralis; 2, nativa; 3, britovi; 4, pseudospiralis. The density graph of the predicted (generalized) probability of infection (99% interval) is also shown.
The grey area in Figure 1 is a density graph of predicted infectivities, generalizing over all included outbreaks. This distribution is obtained by sampling from the ‘group’ (prior) distributions for the infectivity parameters α and β and it represents the infectivity of an unspecified Trichinella inoculum with properties as described by the collection of outbreaks used. This distribution may be used for prediction of the risk of infection.
For the predicted dose–response relationships the inoculum is assumed to be Poisson (i.e. no extra-Poisson variation, or dispersion ρ≫10). Of course, for any particular exposure scenario assuming dispersion, simulations may include any degree of dispersion required.
Figure 2 shows a density graph of the distribution of p m, the survival probability of individual larvae. The shading corresponds to that in Figure 1.

Fig. 2. Density graph of the single-hit probability of infection: distribution (beta probability density) of p m. As in Figure 1 shading corresponds to density, darkest region close to the median and outer margins spans a 99% interval.
Metrics derived from the fitted outbreak dose–response model are listed in Table 3. These illustrate in particular the low dose behaviour of the model: as the single-hit probability p m is ~0·01, exposure to exactly one male and one female larva is expected to lead to infection with a probability of 0·01×0·01=10−4. Although the infection risk at high doses (>10 larvae) is considerable, with decreasing doses the risk rapidly decreases, so that at a dose of 0·1 (approximate exposure to one larva in each 10 exposure events) the risk is quite low (97·5% level near 1/10 000).
Table 3. Distributions of two metrics of Trichinella infectivity: (a) ID50 and ID1, and (b) the probability of infection at various (low) doses
Dose is expected number of larvae (of either sex), calculated as concentration (larvae/g) times intake of contaminated food (in g), as in equation (1).
DISCUSSION
Prime evidence for the human health effects of any infectious microorganism is found in outbreaks. If outbreak studies succeed in obtaining data on exposure of cases, they may be interpreted as ‘natural experiments’, and may be used for dose–response assessment. Compared to previous studies of bacterial infectivity based on human outbreak data [Reference Teunis, Takumi and Shinagawa2, Reference Teunis, Ogden and Strachan3, Reference Teunis15], Trichinella is interesting because infection requires the presence of both a female and a male parasite. The necessary adaptations to the mathematical model used for dose–response assessment cause the relationship to change shape. Compared to a ‘non-sexual’ dose–response model the relationship is steeper, dependent on the sex ratio of the parasites. The parametrization of the dose–response model represents useful a priori information when data are not very informative regarding the shape of the relationship [Reference Teunis and Havelaar4].
Based on the collection of outbreaks we concluded that infectivity of Trichinella in humans is high, ranging from a likely value of 0·01 to an upper (95%) level near 0·1 (Fig. 2): only a few pairs of Trichinella larvae are needed to achieve a considerable probability of infection. The median 50% infectious dose is close to 150 larvae (Table 3 a).
As a consequence, safe levels of Trichinella larvae in meat corresponding to acceptable risk levels should be low, e.g. limiting the average individual yearly dose to <0·1 (thereby limiting individual yearly risk to about <1/10 000). For any single exposure event, even ingestion of a few Trichinella larvae already represents a considerable risk of infection of a few per cent (Table 3 b).
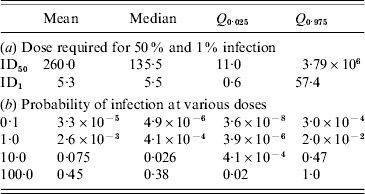
Routine testing surveillance in The Netherlands consists of taking pooled samples of 1 g from 100 slaughtered pigs and inspecting these for the presence of Trichinella larvae after digestion of the meat tissue. Assuming the digestion test has a probability of failing [varying as a beta distribution with parameters (α, β)=(1·0, 1·5) so that the average sensitivity is 40%] and a heterogeneous (gamma-distributed) concentration of larvae in meat, the infection risk associated with consumption of 100 g undercooked meat may be calculated, as a function of the numbers of meat samples analysed. With increasing numbers of samples, the risk rapidly decreases to low (acceptable) levels, as shown in Table 4. In the past years, millions of meat samples have been tested, therefore infection risks are low.
Table 4. Simulated infection risk when standard surveillance of testing 1 g of meat by digestion and microscopic inspection is done for increasing numbers of samples (N), producing only negative results. The digestion test is assumed to fail with a mean probability of 0·4 (95% CI 0·016–0·915). Exposure is estimated for a single portion of 100 g undercooked meat
In contrast to waterborne exposure, pathogens in food cannot usually be assumed to be well dispersed. In agreement with methods reported in an earlier paper [Reference Teunis, Ogden and Strachan3], we estimated average doses from the average concentration of larvae in the foodstuff and the average quantity consumed. All heterogeneity in exposure, due to uneven distribution of larvae in the foodstuff as well as variation in the consumed portions in human cases, was summarized in a dispersion coefficient (ρ). Such dispersion also is a priori information that can be inserted into the dose–response relationship, helping to determine its shape.
Compared to a simple, straightforward dose–response assessment we have accounted for three sources of variation: (1) requirement of sexual reproduction for infection; (2) overdispersion in exposure due to inhomogeneous distribution of larvae and variable consumption; and (3) variation in infectivity among outbreaks, due to different Trichinella spp., differences in susceptibility of cases and other (unspecified) variation in exposure. The latter factor is accounted for by the hierarchical structure of the model.
Although in all outbreaks the infected meat must have contained viable larvae, some die-off may have occurred. In one of the outbreaks [Reference Gari-Toussaint7] the meat had been frozen at −35°C for 7 days, and most of the other incidents involved heating. Doses may therefore have been overestimated if the larvae were counted in a sample of the raw meat instead of the consumed meal. That would mean that the infectivity estimates would be biased downwards. As our analysis indicates very high infectivity, the error caused by underestimation of the dose is not likely to be considerable.
Detection of asymptomatic cases in outbreaks is usually a difficult problem. In most of the selected outbreaks used here, blood samples were collected, not only from cases, but also from all exposed subjects. This allows the use of seroconversion as an indicator of infection, including asymptomatic infections. It appears that the fraction of infected cases that were symptomatic is quite high: 99% (212/214), indicating that the conditional probability of becoming ill when infected with Trichinella may be very high. Therefore, our dose–response model does not distinguish between symptomatic and asymptomatic infections, assuming all infections are symptomatic.
Although animal models of infection and disease are important in clinical research, dose–response studies with animals are not well suited for human risk assessment. The purpose of a dose–response model is not just to establish a causal relationship between dose and probability of infection, but also to quantify that probability. Even when an animal model shows an appropriate response (e.g. comparable symptoms), there is little reason to trust that the magnitude of such a response would be the same as in a human host.
An experimental study of Trichinella infection in pigs [Reference Nöckler16] showed lower infectivities, indicating that humans may be more susceptible to infection with this parasite than pigs. Recent experimental infections in rats seeking to establish the dose–response relationship [Reference Takumi17] also showed high susceptibility, different from pigs. In pigs, different infectivities have been found for different Trichinella spp. [Reference Kapel18–Reference Kapel20]. Human susceptibility to symptomatic infection was high in all observed outbreaks, in spite of the fact that these involved various species of Trichinella. Little variation may be seen if the susceptibility to each species is so high that infection is probable, given even minimal exposure [Reference Teunis and Havelaar4]. We recognize the possibility, however, that the actual range of infectivity among and within these parasite species may exceed that represented in the present sample of highly infectious types.
Outbreaks caused by highly infectious, highly pathogenic parasites are more likely to be detected than those caused by milder parasites. The selection of outbreaks used for dose–response assessment may therefore be biased towards those more infectious parasites, possibly overestimating the risk in the general population. The collected outbreaks showed a variety of infectious Trichinella spp., so that use of outbreak-based infectivity estimates seems a prudent approach, using the precautionary principle of risk assessment [Reference Krewski21].
The dose–response model developed here may be used either in studies of human risk of trichinellosis or to assess testing criteria for slaughtered animals to prevent human cases. Exposure estimates can be translated into estimated probabilities of infection and illness, or projected numbers of cases for specific exposure scenarios can be calculated. For this purpose, the predicted dose–response relationships (the grey area in Fig. 1) may be used, as they represent the infectivity of a hypothetical isolate, i.e. a sample from a population like the selected collection of outbreaks. The present study is based on a relatively small number of outbreaks, not allowing stratification for pathogen type (species), food type (preparation, storage) or host properties (age, proxies for immune competence). To further specify the dose–response relationship of Trichinella in humans, more outbreak data are needed, preferably with reliable information on the numbers of larvae present in the contaminated food.
It can be argued whether the current method of testing is sufficiently sensitive to prevent human cases. Currently, susceptible animal species destined for human consumption must be tested for the absence of Trichinella spp. at slaughter or at game-handling plants in the European Union (EU regulations). The method prescribed for routine testing of pig meat is artificial digestion, which is typically applied by pooling up to 100 samples of at least 1 g meat. However, this reference test has limitations in terms of diagnostic and analytical sensitivity and it was demonstrated that reliable detection of Trichinella-positive samples was only guaranteed when samples contained >3 larvae/g [Reference Forbes and Gajadhar22]. For this reason meat from pigs testing negative might be infected with higher numbers of larvae than are considered safe for consumption. Our dose–response model even shows that the consumption of 100 g pork infected with 200 larvae might test negative by the approved method but can be considered unsafe for human consumption since the probability of disease after ingestion of 200 larvae is considerable. Based on the present study, it may be prudent to recommend reconsideration of the current testing protocol.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
The contribution of P.T. to this study was funded by POLYMOD, a European Commission project funded within the Sixth Framework Programme (contract no.: SSP22-CT-2004-502084). The contributions of J.vd.G. and K.T. were supported by the Network of Excellence MedVetNet (WP TrichiMed) within the Sixth Framework Programme of the European Union and by the Inspectorate of Health Protection and Veterinary Public Health, Food and Consumer Product Safety Authority (VWA), The Netherlands. We gratefully acknowledge the help of our colleagues Karsten Nöckler (Federal Institute for Risk Assessment, Berlin, Germany) and Jean Dupouy-Camet (Laboratoire de Parasitologie–Mycologie, Université R. Descartes, Paris, France) with collecting outbreak data and helpful discussions. Two anonymous reviewers are thanked for their scrutiny and helpful suggestions that helped to improve this paper.
DECLARATION OF INTEREST
None.








