INTRODUCTION
Hospital-acquired infections (HAIs) represent a major issue for health providers, infection control specialists, hospital managers, patients and public authorities. Prevalence surveys show that in Europe, between 3·5% and 9·0% of patients in acute hospital settings are infected [1]. Not all HAIs are preventable but preventive measures can result in a wide range of relative reductions in infection rates, between 10% and 70% depending on the setting, study design, baseline infection rate and type of infection [Reference Harbarth2]. These infection control measures have an associated cost, therefore the expense of an infection control programme should be compared with the expected benefits, ensuring that the most cost-effective measures are implemented [Reference Graves3]. The first step in the economic evaluation of prevention measures is to assess the overall burden of disease, in terms of excess deaths, hospitalization days and costs. This step is a challenge on its own and previous studies in this area were often restricted to the intensive-care unit (ICU) or a specific type of HAI.
These restrictions and the fact that there were no data for the overall burden of HAIs in Belgium, motivated us to perform this study which was divided into three phases (Fig. 1). In the first phase, a nationwide prevalence study was organized and half of Belgian hospitals participated. More than 17 000 patients were surveyed in October 2007 and the study showed a 6·2% prevalence of patients infected [Reference Gordts4]. In the second phase, a matched cohort study was performed using cases identified in the prevalence survey and control patients selected from hospital administrative databases. This second phase aimed to estimate excess mortality and length of stay (LoS) for all types of infections, per type of infection outside the ICU setting. In the third phase the results from earlier phases were used along with data from the Belgian Institute of Public Health (IPH) national surveillance of HAIs in ICUs, combined with other external data, to estimate the annual excess mortality attributable to HAIs, the number of bed-days lost to infection and the total economic cost of infection to the public healthcare provider.
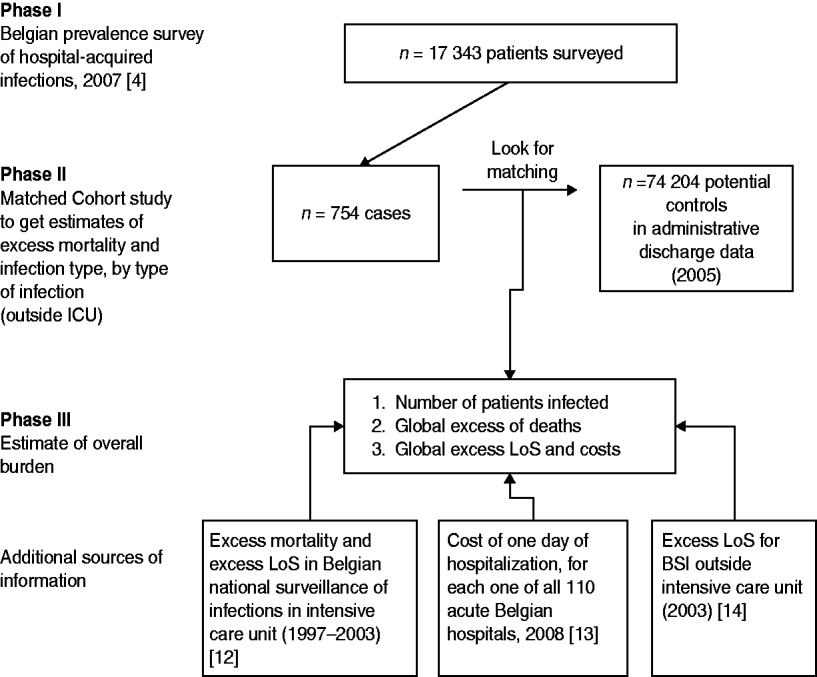
Fig. 1. Study design by phase, and details of external sources of information. LoS, Length of stay; BSI, bloodstream infection.
METHODS: PHASE II
Selection of cases
The HAI cases were identified during a national point prevalence survey in 2007, which used a rule-based expert system implementing Centers for Disease Control and Prevention (CDC) criteria. This survey covered all types of infections: urinary tract infections (UTIs), surgical site infections (SSIs), bloodstream infections (BSIs), lower respiratory tract infections (LRIs) and gastrointestinal infections (GIs), as well as less frequent infections (grouped under the category ‘other’). A total of 63 acute-care hospitals, representing about half of Belgian hospitals, participated in the survey [Reference Gordts4].
Although all wards were included in the survey, data from patients surveyed in ICUs could not be used for matching because detailed daily data were not available in this study. Maternity, neonatal care, paediatric wards, and psychiatric wards were also excluded from Phase II because they represented small heterogeneous groups of patients [Reference Gordts4].
After approval by the Belgian privacy commission, the clinical hospital discharge administrative data for the HAI cases were collected 3 months after the survey by a trusted third party, recoded and transferred for analysis. These data included the year of birth, sex, LoS, date of death if applicable, primary diagnosis of hospitalization, comorbidities and procedures coded with International Classification of Disease, 9th revision (ICD-9) codes. All stays were also classified by all-patient refined diagnosis-related group (APR-DRG).
Selection of control patients
Clinical hospital discharge administrative data from 2005 were used for the selection of controls, because it routinely takes about 2 years before these data are available for analysis. As there is currently no algorithm which allows identification of patients with HAI in administrative data, it was not possible to exclude these patients from the control group.
Matching factors
Each case was matched to 1, 2, 3 or 4 control(s), depending on the number of available controls. Two matching procedures were performed: first for the analysis of excess mortality, and then for the analysis of excess LoS, excluding from the cases and control group all patients who died during hospitalization. Six matching factors were used: hospital, APR-DRG, age (allowing for a maximum difference of 15 years between a case and a control), ward (geriatric, rehabilitation or other), Charlson score (a proxy of patient comorbidity), and the estimated LoS prior to infection. The choice of each factor was justified based on previous research [Reference Haley5–Reference Orsi8]. For the analysis of the extra LoS the destination after discharge (home or elderly residential care) was also added as a seventh matching factor. This was used as a proxy for the residence of the patient before hospitalization, as this latter variable was not readily available for analysis, and previous analyses had shown that it was a confounding factor as patients discharged to residential care had longer LoS and a higher risk of HAI. Gender was not a matching factor.
As exposure duration was not recorded during the 2007 survey, it was assumed that each patient with a HAI was halfway through the infection. The standard duration of treatment was used as a proxy for infection duration: 10 days for most infections, with the exception of UTI (5 days), infections of eye, ear, and mouth (5 days) and infections of bones and joints (20 days). For example, a patient was surveyed the 30th day of his stay and had a nosocomial BSI; this 30th day was then assumed to be the fifth day of the infection (total duration of 10 days), implying that the exposure duration of this patient was 25 days. Controls for this patient were thus selected from those staying at least 25 days in the hospital.
In the matching procedure, patients suffering from several infections at the date of the survey (12% of all cases) [Reference Gordts4] could only be matched once, and were therefore categorized based on what was deemed to be their main infection. BSI, and then LRI were considered the main serious infection if present. Patients with multiple infections not involving a BSI or a LRI were categorized in the ‘other’ group.
Excess in-hospital mortality
For the assessment of excess in-hospital mortality per infection type, conditional logistic regression models were used to account for the different numbers of controls per case. The absolute risk difference (ARD) of mortality between the two groups was used to estimate excess mortality.
Excess LoS per infection type
All analyses on excess LoS were based on patients surviving hospitalization. The mean LoS was first computed for each set of control patients matched with a single case, and then the difference between each case-group of controls was computed with 95% confidence interval (CI) values around the mean. Sensitivity analyses were performed for different assumptions on the duration of the ongoing infection and for matching using less or more of the matching variables, including gender.
With 700 infected patients, this study had 95% power to detect a difference of 4 days in LoS between the groups of infected patients and control patients based on a type I error of 5% and a standard deviation of the difference in LoS of 30 days. As this was the primary objective, no sample size calculations were performed to detect differences in mortality between the two groups.
All analyses were performed with SAS v. 9 software (SAS Institute Inc., USA). The matching algorithm used a publicly available SAS macro developed by the Mayo clinic [Reference Bergstralh and Kosanke9].
RESULTS: PHASE II
Description of cases
Administrative hospital discharge data were received for 754 cases (Table 1) from five distinct wards: surgical (244 patients), medical (245), mixed surgical/medical (26), geriatric (148) and rehabilitation (91). The infections most prevalent were UTIs (26·7%) and LRIs (15·7%). The median age of cases was 74 years, the median total LoS of infected patients was 41·5 days and the median time already spent in hospital was 22 days at the day of the survey. A total of 12·6% of the patients infected died during their hospitalization. Mortality was the highest for patients with LRIs (23·7%), BSIs (15·0%) and GIs (14·5%).
Table 1. Phase II: description of patients with hospital-acquired infection, outside the ICU setting, by major site of infection
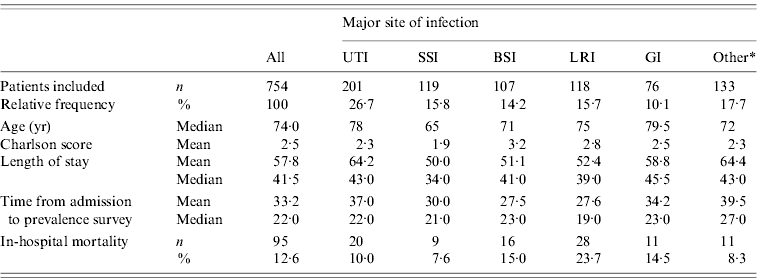
UTI, Urinary tract infection; SSI, surgical site infection; BSI, Bloodstream infection; LRI, lower respiratory tract infection; GI, gastrointestinal infection.
* Other infection types include infections of: skin and soft tissue (n=52), multiple site of infections (n=34), bones and joints (n=17), eye, ear and mouth (n=13), reproductive system (n=6), upper respiratory tract (n=6), cardiovascular system (n=3) and central nervous system (n=2).
Excess in-hospital mortality
A total of 585 cases out of 754 could be matched to 1926 controls (77·6% of cases included, matching ratio 3·3). In-hospital mortality (Table 2) was 12·8% in the infected patient group and 10·8% in the control group after adjustment for the different numbers of controls per case. The overall excess mortality was 2·0% (95% CI−1·7 to 5·7), and varied according to the type of infection, with the strongest absolute effects observed for LRI (9·6%) and BSI (6·1%). Surprisingly, negative estimates of excess mortality were observed for the UTI group (−1·4%) and for the ‘other’ group (−5·4%).
Table 2. Phase II: effect of hospital-acquired infection (HAI) on in-hospital mortality, by major site of infection
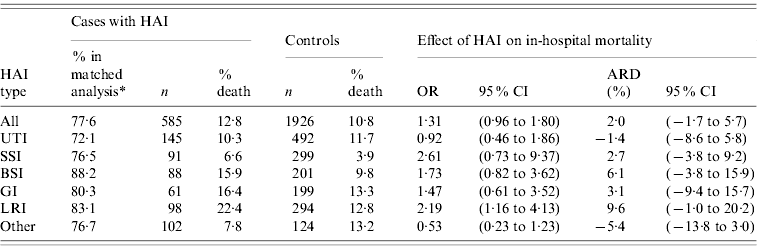
OR, Odds ratio; CI, confidence interval; ARD, absolute risk difference; UTI, urinary tract infection; SSI, surgical site infection; BSI, bloodstream infection; GI, gastrointestinal infection; LRI, lower respiratory tract infection.
* %=number of patients included in matched analysis related to the number of patients in study.
Excess LoS
Of the 659 patients who were discharged alive, 445 (67·5%) could be matched to 1381 controls (matching ratio 3·1). Overall, the mean excess LoS was 7·8 days (95% CI 5·1–10·5), and varied per type of infection from 4·6 days for UTIs to 12·1 days for GIs (Table 3). Distributions of excess LoS were all skewed, as indicated by smaller medians (and the minimum of 0·5 days for UTIs).
Table 3. Phase II: effect of hospital-acquired infection (HAI) on length of stay (LoS), by major site of infection
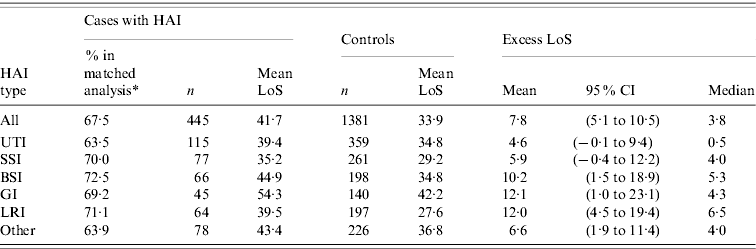
CI, Confidence interval; UTI, urinary tract infection; SSI, surgical site infection; BSI, bloodstream infection; GI, gastrointestinal infection; LRI, lower respiratory tract infection.
* %=number of patients included in matched analysis related to the number of patients discharged alive in study.
Sensitivity analyses of the matching factors showed that the exposure duration was the most important factor influencing the estimates of excess LoS, and that once this had been accounted for, other factors (age, Charlson score, ward, destination after discharge) played a limited role (Table 4). This excess LoS was reduced by a factor of 4 when exposure duration was taken into account: from 39·4 days when only hospital and APR-DRG were matched, to 9·8 days when exposure duration was included. When the convention used to determine exposure duration was varied by 2 days (assuming 8 days or 12 days instead of 10 days for the duration of infection), the resulting mean excess LoS varied by a maximum of 1 day.
Table 4. Phase II: sensitivity analyses on the choice of different matching factors effect of hospital-acquired infection on length of stay
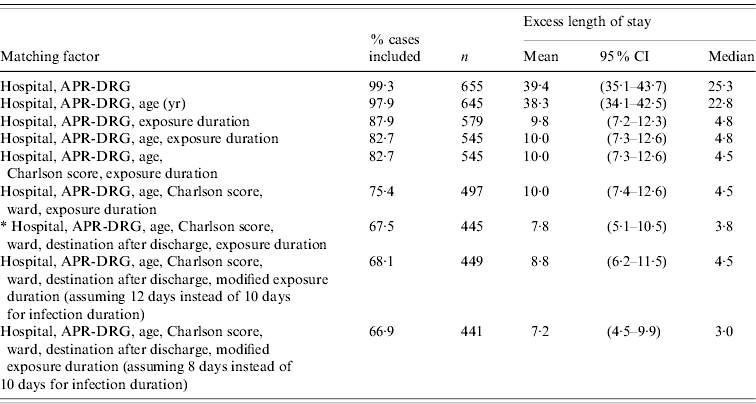
CI, Confidence interval; APR-DRG, all patient refined diagnosis-related group.
* Final model presented in Table 3.
METHODS: PHASE III
Number of patients with a HAI
To compute the annual number of patients infected in Belgium, the cumulative incidence (number of patients infected/100 hospital admissions) was required. This information was not available and hence we assumed three scenarios: (a) the cumulative incidence equals the prevalence; (b) the cumulative incidence is lower than prevalence for most infections because some infections are overrepresented in a prevalence survey, as infections theoretically last longer (10 days) than average duration of hospitalizations in Belgium (8·8 days in 2005); (c) the same as scenario b, but accounting for the fact that UTIs have a much shorter duration (5 days) and are hence underrepresented in a prevalence survey. The following conversion factor was used to compute cumulative incidence for each scenario: (a) no conversion factor; (b) 0·88 for all infections (duration of infection, 10 days, divided by average national LoS, 8·8 days); (c) 0·88 for all infections, except UTIs where we used 1·76. This conversion factor was inspired by the formula CI≈P×LA/(LN – INT), where LA is the mean LoS of all patients, LN the mean LoS of infected patients and INT the mean time from start of hospitalization to the infection [Reference Rhame and Sudderth10, Reference Graves11]. We replaced in this formula the values (LN – INT), and thus the number of days between start of infection and end of hospitalization, by the duration of the infection itself. Resulting incidences under each scenario were multiplied by the total number of classic hospitalizations, amounting to 1 869 757 in 2005 (this excludes 1-day admissions) to obtain the total number of patients infected under each scenario. All remaining computations were then based on scenario c, which was the most probable.
Number of excess deaths
For non-ICU patients, the results from Phase II (Table 2, excess mortality) were used. In addition to using negative estimates of excess mortality for UTIs and other infections, which actually imply a protective effect of the infection, no effect on mortality (0%) was also assumed and both estimations are presented.
For ICU patients, the analyses made public by the Belgian IPH were utilized. The excess mortality percentages for BSIs (9·8%) and LRIs (5·7%) were based on a dataset of 1899 cases of BSIs and 5213 cases of LRIs reported by ICUs during the period 1997–2003 [Reference Suetens12]. For the other type of infections in ICU estimates of excess mortality were not found. The number of excess deaths was calculated by multiplying the excess deaths percentages by the number of patients with a HAI under scenario c.
Average cost of one hospitalization day in an acute-care hospital
The cost of one plain hospitalization day (daily cost, i.e. without any medical treatment or diagnostic tests) is available for all acute-care hospitals in Belgium [13]. Instead of using each specific amount for each specific hospital included in our study, we chose to calculate an average cost, based on all hospitals and weighted by their activity volume (in terms of annual number of hospitalization days). This calculation was based on daily costs for 2008.
Number of excess hospitalization days and healthcare provider costs
For non-ICU HAIs, the results of Phase II (Table 3, excess LoS) are the main source for our estimates, except for BSIs, for which results were used from another recent Belgian study, which included a larger number of cases (665 cases infected in 2003) than our matched study [Reference Vrijens14]. The mean extra LoS after a BSI reported in that study was 9·3 days, 1 day less than the 10·2 days of Phase II.
For ICU HAIs, estimates previously reported by the Belgian IPH for mean excess LoS of ICU cases of LRIs (11·4 days) and BSIs (10·2 days) were included [Reference Suetens12]. These are based on excess LoS in ICUs only. For the ‘other’ type of infections in ICUs, estimates derived for non-ICU cases were used.
The number of patients alive was computed based on estimates from Phase II (Table 5, from number of patients with a HAI and in-hospital mortality). The number of excess hospitalization days was computed by multiplying the number of patients alive by the excess LoS for one case, based on different estimates described above. To calculate the cost for the healthcare provider, the excess number of bed-days was multiplied by the average cost of one hospitalization day.
Table 5. Phase III: estimates of yearly number of patients infected, total and excess in-hospital mortality in patients
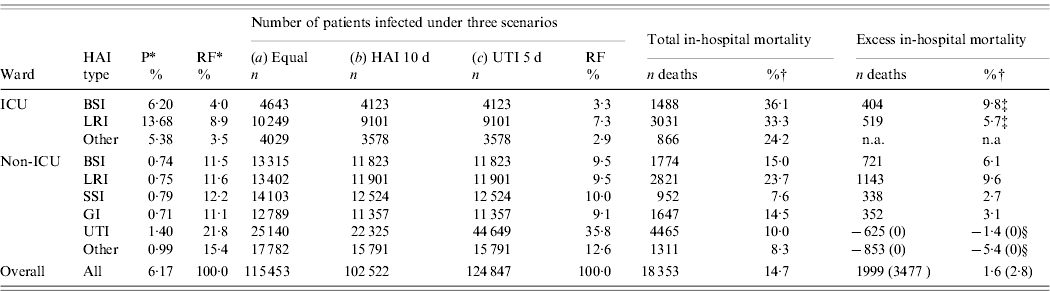
P, Prevalence (per 100 patients); RF, relative frequency; HAI, hospital-acquired infection; UTI, urinary tract infection; BSI, bloodstream infection, LRI, lower respiratory tract infection; SSI, surgical site infection; GI, gastrointestinal infection; n.a., not available.
* From prevalence survey (Phase I).
† All estimates are from Phase II, except where otherwise stated.
‡ From external source of information (Belgian Institute of Public Health, see Fig. 1).
§ Set to 0% because of negative estimates in Phase II.
RESULTS: PHASE III
Number of patients with a HAI
Based on a prevalence of 6·2%, and not adjusting the cumulative incidence (6·2/100 admissions), the number of patients infected was 115 453 (Table 5, scenario a). Using an overall conversion factor of 0·88, the cumulative incidence reduces to 5·456/100 hospital admissions, leading to a total of 102 522 patients infected, of which 22 325 had a UTI (21·1%, scenario b). Taking into account the fact that UTIs last generally half the duration of other infections, this value is doubled to 44 649 patients infected by a UTI (35·9% of infections, scenario c). The total number of patients infected was thus estimated at 124 847, of which 86·5% were infected outside the ICU setting.
Number of excess deaths
About 18 300 patients were estimated to die during hospitalization which was aggravated by a HAI. Using negative estimates of excess mortality for UTIs and other infections, the number of excess deaths was 1999 (1·6%). Using the more realistic assumption of no excess mortality for UTIs and other infections leads to a total of 3477 deaths (or 18·9% of all deaths) which can be attributed to HAIs. Overall excess mortality in the 124 847 patients with a HAI was thus 2·8%, as detailed in Table 5. Excess in-hospital mortality in non-ICU wards was estimated at 2554 deaths per year (73·5% of all excess deaths).
Average cost of one hospitalization day in an acute-care hospital
The average cost of one hospitalization day in an acute-care hospital was €371 in 2008.
Number of excess hospitalization days and healthcare provider costs
Table 6 presents the overall estimates for excess LoS and cost. On a national scale, the number of hospitalization days attributable to HAIs was estimated at around 780 000 days, or 7·3 on average per infection. Taking into account the cost per hospitalization day this amounts to an excess of €290 million for the public healthcare provider. Costs outside ICUs represented 14·1% of this amount.
Table 6. Phase III: estimates of yearly excess in-hospital stay and healthcare provider costs
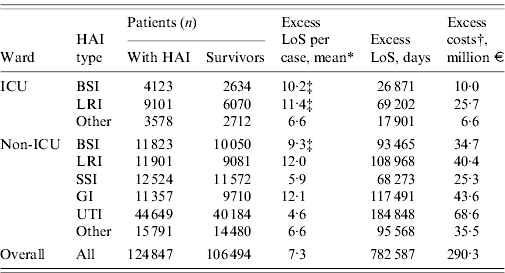
HAI, Hospital-acquired infection; LoS, length of stay; BSI, bloodstream infection; LRI, lower respiratory tract infection; SSI, surgical site infection; GI, gastrointestinal infection; UTI, urinary tract infection.
* All estimates are from Phase II, except where stated otherwise.
† Based on a cost per day of €371.
‡ From an external source of information (see Fig. 1).
DISCUSSION
We estimate that each year HAIs affect 125 000 patients in Belgium, and that 3500 die in hospital due to this infection. A total of 800 000 excess days of hospital stays leads to an extra public healthcare cost of €290 million. These are the first published estimates on the annual burden of infections for Belgium at a national level.
Very few comparable studies have been done at a national level. Our methodology is similar to that of a recent US study [Reference Klevens15], in which the authors attempted to make the best use of existing national data, by using a multi-state approach and pooling estimates of attributable mortality from three national databases. They estimated that 1·7 million patients are infected every year, and that 100 000 deaths could be attributed to HAIs. We can only agree with their conclusions, that ‘these estimates are sobering and reinforce the need for improved prevention and surveillance efforts’. Another UK study estimated that 320 994 patients in England acquire a hospital infection every year, and that these infections cost £930·62 million to the hospital sector [Reference Plowman16].
The overall estimate of the excess LoS, based on surviving patients, was on average 7·3 days, which is almost twice the seminal estimate of 4 days from the 1981 study of Haley et al. [Reference Haley17], but lower than other published estimates [Reference Plowman16]. For SSIs, our estimate of 5·9 is at the lower end of the published values, which may be explained by the trend towards earlier hospital discharge. It should be noted that the costs of SSIs occurring or being treated in the community after the hospital stay are not included in our estimates. Finally, the average estimate of 4·6 additional days for a UTI seems high, but might be the result of rather complex cases in elderly patients surviving a prolonged hospitalization, mainly on geriatric or rehabilitation wards.
The results also show that the burden of HAIs in terms of mortality and costs for ICU patients is large but in absolute numbers it is much larger for non-ICU wards. Seventy-three percent of attributed deaths, 87% of global costs and 87% of patients infected were outside the ICU setting.
These estimates have several limitations. The first relates to the lack of global national incidence data: as there is no general continuous surveillance system of HAIs in Belgium, we had to rely entirely on data from the only recent existing prevalence survey to calculate the annual number of patients infected. We corrected as much as possible for over- and underrepresentation of infections in the prevalence survey due to differences in infection duration, being well aware that there is no validated formula to do this [Reference Gastmeier18]. Nevertheless, we considered that a specific correction had to be used for UTIs, which had no effect on mortality. This correction resulted in doubling the relative frequency of UTIs in the national estimates compared to those observed in the prevalence survey. The choice of the average duration of 10 days for all HAIs and 5 days for UTIs is arbitrary and could be challenged. Our results are sensitive to this arbitrary choice.
A second limitation of our analysis is the way in which estimates of attributable mortality and LoS from a matched cohort study were derived. This is a rather old methodology, as new analysis techniques based on competing risks in the framework of time-to-event data are slowly becoming the new standard, as shown in the recent BURDEN study [Reference Lambert19]. These methods also allow accounting for the effects of patients dying in hospital, while we opted to study the effect on hospital stay only for patients surviving the infection, because it was our intention to separate explicitly the effect of infections on mortality and LoS, as death due to the infection may lead to shortened hospital stay. Nevertheless, these new methods require that all relevant variables are recorded daily in a standard way, which is rarely the case on standard hospital wards. Most of this research is therefore limited to ICUs. As our analysis is based on administrative data, detailed daily information was not available and we choose a matched cohort design. The two known problems with such a design are the exclusion of unmatched patients from the comparison and the overestimated results. We attempted to overcome the latter by matching for time before infection, and demonstrated that matching for the length of hospital stay prior to the HAI is crucial for obtaining credible estimates for excess mortality and LoS in patients infected by a HAI. The importance of this adjustment can thus not be overstated. Some authors compared this approach with approaches based on time-to-event, and concluded that it can lead to an estimation twice as large [Reference Schulgen20]. In our case, dividing excess costs by half still results in a sum of €150 million, which remains a significant public healthcare cost.
Lack of detailed clinical information and daily information (data are summarized at discharge) explains why it was not possible to find appropriate control patients for HAI cases in ICUs, and we had to rely on information published by the Belgian IPH. Another option would have been to use peer-reviewed international estimates for excess mortality and LoS in ICUs, but it was considered that the study results might have more impact on Belgian decision-makers if all source data were national. In addition, the delay in access of administrative data necessitated the use a historical control group, without any information of the infection status and under the untested assumption that hospital discharge practices did not change over the 2-year period. On the other hand, the use of a large set of administrative data to select appropriate control patients permitted the assessment of the sensitivity of the choice of matching factors, and allowed inclusion of a relatively high number of cases in the analysis. Cases that could not be matched are more likely to represent unique patient hospital stays, and the impact of not including these remains unclear.
The last limitation relates to the use of the charge of one hospitalization day to estimate national costs for the third-party funder. In addition, costs of diagnostic techniques, pharmaceutical products and interventions should be added to this burden, but collecting this detailed information was considered beyond the scope of this project.
In this report, we estimated the burden of HAIs in terms of extra bed-days and the related gross charges from a public healthcare provider's perspective. It should be noted that preventive measures can only in part eliminate HAIs and their excess costs. In addition, policy-makers should take into account that any reduction of LoS because of preventive measures will lead to a more efficient use of resources in the short term, without necessarily impacting on the overall healthcare expenditure. Evaluating the economics of preventing HAIs from a hospital perspective or from a public funding perspective are two separate questions. Excess costs estimated for HAIs should definitely not be interpreted as cash which would become available in the short term if some HAIs were prevented. These considerations should, however, not cast any doubt on the desirability of avoiding HAIs.
ACKNOWLEDGEMENTS
The authors thank Dr Bart Gordts, Dr Chris de Laet and Mr Gert Peeters. The authors also thank the anonymous referee for very constructive comments, which helped to improve clarity of the manuscript.
DECLARATION OF INTEREST
All authors are employed by the Belgian Health Care Knowledge Centre.









