INTRODUCTION
Campylobacter is a common cause of bacterial foodborne illness in the developed world [Reference Rocourt1, 2]. Infection with Campylobacter typically causes diarrhoea (often with blood), vomiting and other gastrointestinal symptoms lasting for between 2–7 days; however, more severe illness and long-term complications also occur [Reference Havelaar3, Reference Helms4]. Campylobacter resides in the intestinal tract of many wild and domestic animals, particularly birds, and hence infection can occur following contact with these animals [Reference Savill5, Reference Broman6]. Most commonly humans become infected by consuming food (particularly meat, poultry, and milk) or water that has been contaminated by the faeces of animals, or cross-contaminated from contact with other contaminated foods [Reference Neimann7–Reference Friedman9]. The incidence of human Campylobacter infections is seasonal, peaking in the spring and summer, suggesting that the sources of infection may vary seasonally [Reference Kovats10, Reference Tam11].
There have been efforts to better describe the epidemiology of campylobacteriosis internationally and some similarities have emerged, including very consistent seasonal patterns from year to year [Reference Nylen12]. However, there is also marked variation in the reported incidence of culture-confirmed Campylobacter infections between some countries [13–15]. In the USA, the reported incidence of culture-confirmed infections ascertained by active surveillance ranges between 10 and 30 cases/100 000 persons per year, whilst in Australia it ranges between 100 and 200 cases/100 000 persons per year [13, 15]. The reasons for the disparity between the two countries are unknown. Stool specimens submitted to laboratories are routinely tested for Campylobacter in both countries [Reference Hall, Raupach and Yohannes16, Reference Voetsch17] but there could be other differences in healthcare systems that may contribute to this disparity. Differing healthcare systems may influence reported incidences of culture-confirmed Campylobacter infections by impacting on medical care-seeking behaviours or the frequency with which stool specimens are cultured by clinical laboratories. To explore this, we used surveillance data and other epidemiological studies to examine whether the rate at which individuals presented to medical officers and submitted a stool specimen may account for the disparity in reported incidences of Campylobacter infection in Australia and the USA.
METHODS
Incidence of culture-confirmed Campylobacter infection
Australia
The Commonwealth Department of Health and Ageing established the OzFoodNet network to enhance surveillance for foodborne disease across Australia in 2000 [Reference Ashbolt18]. OzFoodNet network partners include the National Centre for Epidemiology and Population Health at The Australian National University, the Public Health Laboratory Network, and all eight states and territories of Australia. Doctors and laboratories in Australia in all jurisdictions are required by law to report culture-confirmed cases of Campylobacter infection to the relevant state and territory health departments in Australia, with New South Wales being the only exception (Fig. 1). In Western Australia legislation requiring laboratories to notify was enacted in 2006 but doctors were mandated to report prior to this and most laboratories were notifying culture-confirmed cases under a voluntary arrangement. The incidence of culture-confirmed Campylobacter infection for Australia in 2001 was estimated by aggregating data for the seven notifying Australian jurisdictions and dividing by the census population of 12 850 965 for these seven sites. Age-specific culture-confirmed Campylobacter incidences were also calculated along with incidence by state.
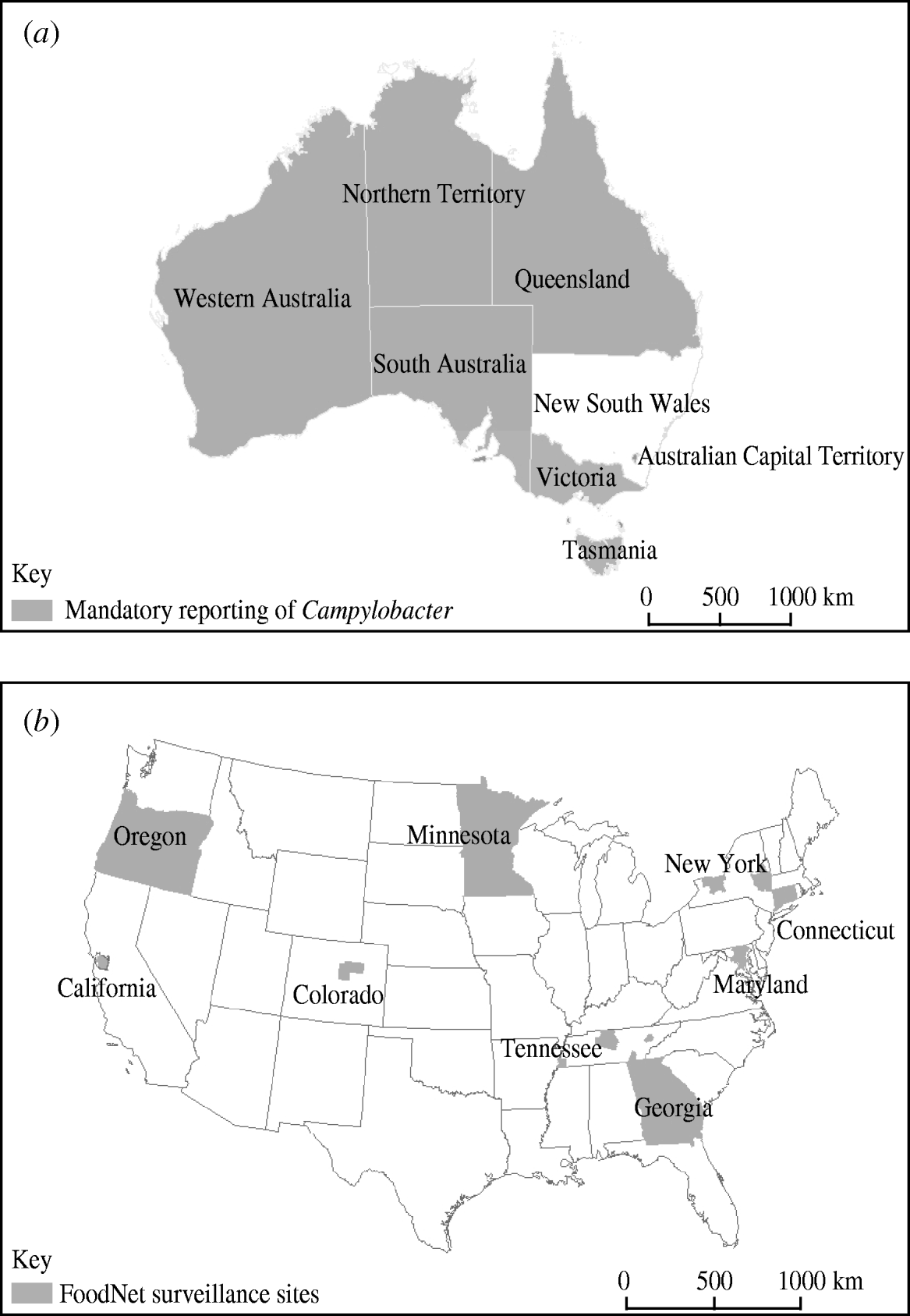
Fig. 1. Surveillance for Campylobacter by (a) OzFoodNet in Australia and (b) FoodNet in the USA in 2001.
USA
The Centers for Diseases Control and Prevention established the Foodborne Diseases Active Surveillance Network (FoodNet) for enhanced surveillance of foodborne diseases in the USA in 1996 [Reference Scallan19]. FoodNet partners include the U.S. Department of Agriculture, the U.S. Food and Drug Administration, and participating state health departments. FoodNet personnel regularly contact clinical laboratories to ascertain all culture-confirmed Campylobacter infections in residents of the FoodNet catchment area. Clinical laboratories records are audited twice a year to ensure complete case ascertainment. In 2001 there were nine FoodNet sites, including Georgia, Minnesota, Oregon, and selected counties in California, Colorado, Connecticut, Maryland, New York and Tennessee (Fig. 1). The incidence of culture-confirmed Campylobacter infections in the USA in 2001 was estimated by aggregating data for each of the nine FoodNet sites and dividing by the census population of 34 900 764. Age- and state-specific culture-confirmed Campylobacter incidences were also calculated.
Rates of seeking medical care and submitting a stool sample
Cases ascertained through laboratory-based surveillance represent only a subset of Campylobacter infections in the community. A number of events or surveillance steps must occur before a culture-confirmed case is ascertained, including that the ill person must seek medical care and submit a stool specimen. Estimation of the probabilities of seeking healthcare and submitting a stool specimen were made from results of population-based telephone surveys on gastroenteritis that were conducted in Australia and the USA [Reference Scallan20]. In both surveys, persons ill with diarrhoea (defined as ⩾3 loose stools in any 24-h period in the previous 4 weeks and excluding those with diarrhoea due to chronic illness) were asked if they sought medical care for their illness, and if yes, whether a stool specimen was submitted to a clinical laboratory for culture. Both telephone surveys were conducted over a 12-month period using a similar survey methodology and questionnaire. In the Australian survey, 6087 persons were interviewed between September 2001 and August 2002. In the USA, 14 647 persons in the FoodNet catchment area were interviewed between February 2000 and January 2001. Detailed methods and results for both of these surveys are reported elsewhere [Reference Scallan20].
The likelihood of visiting a doctor and the doctor ordering a stool specimen for submission to a clinical laboratory varies with severity of illness, and in particular, the presence of blood in stool [Reference Scallan21, Reference Hall22]. This is relevant when considering campylobacteriosis, as cases tend to have more severe symptoms than most other forms of gastroenteritis, and a large proportion of cases have blood in their stool. In case-control studies in both countries the symptom profiles for Campylobacter infection appear to be very similar, with a median duration of symptoms of about 6 days, and the proportion of cases having blood in stool in Australia and the USA being 44% and 45%, respectively [Reference Friedman9, Reference Hall, Raupach and Yohannes16]. To take into account the severity of Campylobacter infections in our calculation of indices of healthcare behaviours, gastroenteritis population survey data were stratified into two severity categories based on the presence or absence of blood in stool. For both severity categories, the ratio of the number of cases of gastroenteritis in the community to each case submitting a stool sample was calculated. The overall severity-adjusted ratios were then calculated by weighting the severity-specific ratios according to the proportions of Campylobacter infections that have blood in the stool.
RESULTS
Incidence of culture-confirmed Campylobacter infections by state and age
In 2001, the overall reported incidence of culture-confirmed Campylobacter infections in Australia and the USA was 125 and 14 cases/100 000 persons, respectively, representing about a ninefold difference. In Australia, the annual incidence ranged from 109 cases/100 000 persons in Queensland to 174/100 000 in South Australia (Table 1). Western Australia, where there was possibly incomplete notification of Campylobacter infections by clinical laboratories, had a reported incidence of 137 cases/100 000. In the USA, three States – Georgia, Maryland and Tennessee – recorded the lowest incidence of 7 cases/100 000 persons. The highest incidence was in California with 31 cases/100 000 persons. Age-specific culture-confirmed Campylobacter incidences are presented in Table 2. The highest incidence in both countries was in children aged <5 years (298 cases/100 000 and 27 cases/100 000 in Australia and the USA, respectively).
Table 1. Populations under surveillance, numbers of culture-confirmed Campylobacter infections and crude rates in Australian OzFoodNet sites and U.S. FoodNet sites, 2001
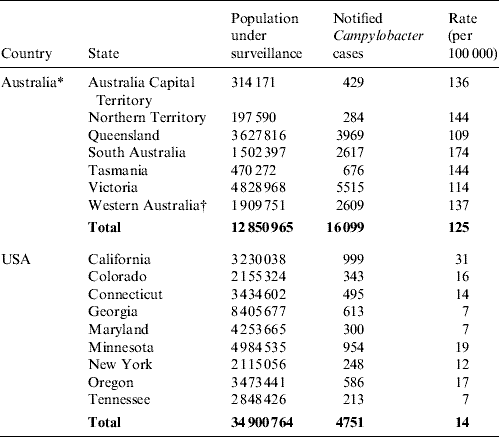
* New South Wales is not included in this table as Campylobacter infections are not notifiable in this Australian state.
† Laboratory notifications were not mandatory in Western Australia in 2001.
Table 2. Populations under surveillance, numbers of culture-confirmed Campylobacter infections and age-specific Campylobacter incidences in Australian OzFoodNet sites and U.S. FoodNet sites, 2001

* Excluding New South Wales.
Adjusting for differences in seeking medical care and providing a stool specimen
Across the two countries there was some variation in the probabilities for seeking medical care and submitting a stool specimen within the severity categories based on the presence or absence of blood in stool. However, the severity-specific ratios that account for both the steps of seeking medical care and ordering a stool test (i.e. the ratio of community cases to cases providing stool specimens) were more similar. For diarrhoea cases with blood in the stool, the estimated ratios were 4:1 in both countries (Table 3). For cases without blood in the stool the ratios were 29:1 and 23:1 in Australia and the USA, respectively. The severity-specific ratios were weighted by the proportion of Campylobacter cases with blood in the stool to calculate an overall ratio. This suggested that for each Campylobacter case providing a stool specimen there were 18 cases in the community in Australia and 14 in the USA. This represents a 1·3-fold differential across the countries, indicating fairly similar practices of seeking medical care and stool-culture ordering for severe gastroenteritis typical of campylobacteriosis.
Table 3. Proportion visiting a medical officer (MO), providing a stool specimen, and ratio of cases providing stool specimen for every community case by severity categoriesFootnote *
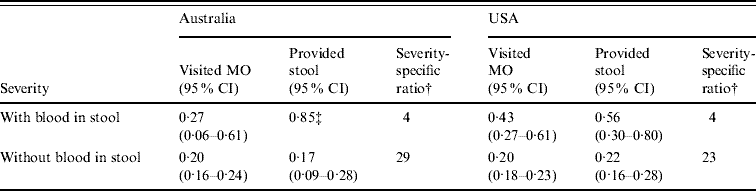
CI, Confidence interval.
* Data from gastroenteritis surveys in both countries [Reference Hall, Raupach and Yohannes16, Reference Scallan20].
† Ratio of community cases for every stool specimen submitted for culture by clinical laboratories.
‡ Data from General Practitioner surveys on stool-ordering practices from Victoria and South Australia (no confidence intervals available) [Reference Hall, Raupach and Yohannes16].
DISCUSSION
The approximate ninefold higher reported incidence of culture-confirmed Campylobacter infections in Australia than in the USA did not appear to be explained by differences in medical care-seeking behaviour, or the frequency with which stool cultures are ordered by medical practitioners, suggesting that there is a real difference in the incidence of Campylobacter infections between these two countries.
However, it is important to note that two other healthcare system factors need to be considered and may contribute to this difference in Campylobacter incidence. First, differences in the reporting of laboratory-confirmed infections between these two countries may be important. Infectious disease notification from laboratories are mandatory in Australia and are generally programmed into laboratory information systems (R Givney, Hunter Area Pathology Service, personal communication, February 2008), whilst in FoodNet in the USA, surveillance is active [Reference Voetsch17], suggesting a high rate of reporting for both these systems. Second, differences in the frequency with which laboratories test for Campylobacter and their ability to identify Campylobacter in stool specimens may also vary by country, although it seems that laboratories in Australia and the USA almost universally routinely test for the presence of Campylobacter in stool samples [Reference Hall, Raupach and Yohannes16, Reference Voetsch17]. However, laboratory-based differences that may influence sensitivity of testing for this fastidious pathogen may exist. These include variations in specimen transport media, types of media used to culture faecal specimens, times that culture plates are incubated, and how plates are read. At present, laboratory surveys in Australia and the USA are being conducted to more fully understand these possibilities. However, given the enormous differential that currently exists between these two countries in the incidence of reported Campylobacter infection, it seems likely that a difference in incidence will persist after any variation in laboratory practices are taken into account.
Some other limitations must be considered in assessing the findings reported in this study. An important one is the uncertainty in the results due to small numbers in the gastroenteritis surveys, particularly of cases with blood in stool, which is reflected in the wide confidence intervals associated with calculated proportions. It is therefore possible that the severity-specific ratios from the two countries could be more different than the point estimates imply. Another limitation is that whilst the estimate of the incidence of culture-confirmed Campylobacter infections for the USA was obtained from active surveillance in FoodNet sites, the population under surveillance in 2001 represented only ~12% of the population of the USA. However, a demographic examination of the census data for FoodNet sites shows it is similar to the total USA population [Reference Scallan20]. In Australia, New South Wales, the most populous state, in which 40% of the population reside, does not routinely notify culture-confirmed cases of Campylobacter, and thus was excluded from this analysis. A further possible limitation in the analysis was that there was a significant difference between countries in the response rates for the diarrhoeal disease prevalence surveys from which ratios were calculated for healthcare behaviours. The survey in the USA had a response rate of 27%, whilst the survey conducted in Australia had a response rate of 70% [Reference Scallan20]. It is possible that the survey in the USA may have been more biased.
The fact that the ratios used to estimate healthcare behaviours were derived from surveys for diarrhoeal disease and do not relate solely to Campylobacter infection is also a limitation. However, our calculations, taking into account the severity of Campylobacter infections and the influence of the severity of gastrointestinal infections on the probability of seeking medical care and providing a stool specimen, suggest that healthcare behaviours are similar between the two countries for severe cases of gastroenteritis. There was no adjustment for age in these calculations, but we believe that differences in health-seeking behaviours for different age groups were unlikely to account for the considerable difference in the Campylobacter incidence rates between the two countries. The effect of age on the probability of visiting a doctor and ordering a stool specimen is less than the effect of severity, and is similar in the two countries, and is therefore unlikely to have an effect on the disparity we have reported in this paper [Reference Hall, Raupach and Yohannes16, Reference Scallan21]. It is also important to note that this analysis was restricted to surveillance data from 2001 only; however, this was chosen in order to match population-based survey data for gastroenteritis in each country which were used to adjust for seeking medical care and providing a stool specimen.
One possible explanation for the reduced incidence of infection to Campylobacter in the USA is that it is due to increased immunity in this population compared with Australia. However, there is little evidence for this in the literature, and in fact surveillance data presented in this paper argues against this, with the relative rate of infection in young children being greater in Australia. The differing incidence could also be indicative of an unidentified Campylobacter reservoir, or means of transmission, that exists in Australia and not the USA. Alternatively, population-level behavioural differences may explain this disparity. Different food consumption patterns in Australia and the USA, along with different contamination levels of foodstuffs, may result in a higher exposure to Campylobacter and therefore a higher incidence in Australia. Importantly, measured risk factors for Campylobacter infection explain less than 50% of sporadic cases in epidemiological studies [Reference Neal and Slack23, Reference Rodrigues24]. A better understanding of the causes of the difference in incidence between these countries may provide insights into the epidemiology of this pathogen and assist in preventing these infections.
One of the main sources of Campylobacter infections in many countries is contaminated poultry [Reference Friedman9, Reference Stafford25]. Comparative details about the type of chicken consumed in the two countries and the contamination rates of chicken products are not available. However, chicken consumption is high in both countries. In an OzFoodNet study in Australia, 80% of people aged >5 years reported eating chicken in the last 7 days [Reference Stafford26], with a per capita chicken consumption of ~35 kg estimated for 2005 [Reference Trewin27]. In a FoodNet study in the USA, 84% of respondents reported eating chicken in the last 7 days [28], with a per capita consumption of ~27 kg estimated for 2004 [Reference Busby and Farah29]. Similarly, limited microbiological surveys of fresh poultry meat purchased at grocery stores in Australia and the USA report the isolation of Campylobacter in excess of 70% in both countries [Reference Zhao30]. However, no data are available on the relative quantitative counts of Campylobacter found on contaminated chicken in the two countries. Because the risk of infection with Campylobacter will vary depending on the quantitative dose, and the number of Campylobacter organisms on chicken differs between processed poultry meats, such as chicken nuggets, and frozen chicken and fresh meat [Reference Bhaduri and Cottrell31, Reference Wingstrand32], further research is required to explore whether there are differences in the type of poultry consumption, or the degree of contamination of chicken, between Australia and the USA. Variation in cooking styles in the home or commercially between the countries may also be important, with thorough cooking less likely to allow transmission of viable Campylobacter.
It is also important to consider that in Australia and the USA the incidence of culture-confirmed Campylobacter infections varied across jurisdictions (Australia: 109–174 cases/100 000; USA: 7–31 cases/100 000), although these jurisdictional differences were much smaller in magnitude compared with the differences in the incidence between Australia and the USA. California, a state that one might speculate is most similar to Australian states in terms of geography, climate and culture, reported the highest incidence of culture-confirmed Campylobacter infections in the USA. However, it should be noted that this incidence was still some fourfold lower than the incidence in Australia.
In conclusion, the ratios of community cases for every stool specimen provided were similar between countries leading to our assertion that healthcare-seeking behaviour and stool-ordering practices do not explain the disparity in the reported rates of culture-confirmed Campylobacter infection. Consequently, other explanations for the observed difference need to be explored.
ACKNOWLEDGEMENTS
OzFoodNet is funded by the Australian Government Department of Health & Ageing and FoodNet is funded through the Centers for Disease Control & Prevention's Emerging Infections Program. We acknowledge Dr Gary Dowse from the Department of Health, Western Australia and PathCentre, Western Australia for their support of this project. This project was completed as part of the Masters of Applied Epidemiology Program at the Australian National University, which is funded by the Australian Government Department of Health and Ageing. We acknowledge Mr Ivan Hannigan for his assistance with the preparation of the figure for this paper.
DECLARATION OF INTEREST
None.






