INTRODUCTION
Herpes simplex virus (HSV) is the most frequently identified cause of acute infectious encephalitis worldwide [Reference Whitley, Sheld, Whitley and Marra1]. Herpes simplex encephalitis (HSE) presents with a wide range of symptoms making clinical diagnosis difficult. The diagnosis relies on the detection of viral DNA in cerebrospinal fluid (CSF) by a highly sensitive (95–100%) and specific (94–100%) polymerase chain reaction (PCR) test [Reference Whitley, Sheld, Whitley and Marra1, Reference Lakeman and Whitley2]. False-negative PCR results have been observed during the early course of HSE [Reference Weil3, Reference Chaudhuri and Kennedy4], and after more than 10 days of disease evolution [Reference Chaudhuri and Kennedy4, Reference Puchhammer-Stöckl5].
Before the use of antiviral drugs, the case-fatality ratio (CFR) was about 70% [vs. 50% with vidarabine and 14–20% with acyclovir (ACV) treatment] and most surviving patients had severe sequelae [Reference Whitley, Sheld, Whitley and Marra1, Reference Whitley6–Reference Tunkel8]. ACV should be administered as early as possible, even before the laboratory confirmation of the diagnosis, as any delay might result in permanent cognitive or physical impairment [Reference Steiner, Kennedy and Pachner9–Reference Martinez-Torres11]. The usual course of treatment is to give 10 mg/kg t.i.d. of ACV for 2 or 3 weeks [Reference Whitley, Sheld, Whitley and Marra1, Reference Steiner, Kennedy and Pachner9, Reference Poissy10]. However, the marketing recommendations of ACV stipulate a 10-day treatment course in the case of HSE, but also state that the duration of ACV ‘should be adapted to the condition of the patient and response to treatment’. Despite early treatment, some patients still experience a poor outcome.
The usefulness and efficacy of steroids in the treatment of HSE has been under discussion for years and is still a matter of controversy. The GACHE Study currently ongoing in Germany, Austria and The Netherlands is expected to provide answers in the near future [Reference Martinez-Torres11].
We report the clinical profiles of the case-patients identified with HSE and the management of ACV therapy in a prospective study on infectious encephalitis conducted in France in 2007 [Reference Mailles and Stahl12]. The management of ACV treatment is described and discussed with regard to the aetiological diagnosis of patients included in the study, and experts' and manufacturers' recommendations.
MATERIAL AND METHODS
A prospective study was designed to assess the aetiology of infectious encephalitis in France. The case definition of acute encephalitis was a patient aged ⩾28 days, hospitalized in mainland France in 2007 with (1) an acute onset of illness, (2) at least one abnormal result in the CSF (white blood cell count ⩾4 cells/mm3 or protein level ⩾40 mg/dl), (3) fever or recent history of fever ⩾38°C, and (4) decreased consciousness or seizures or altered mental status or focal neurological signs. The protocol for laboratory investigation and aetiology of encephalitis in enrolled case-patients has been described previously [Reference Mailles and Stahl12].
A case of HSE was defined as a patient with HSV DNA detected in the CSF by PCR. If a negative PCR was obtained on a CSF sample taken before day 4 of neurological symptoms, it was strongly recommended to test a further CSF sample for HSV DNA taken on day 4 or later, unless a second lumbar puncture (LP) was contraindicated. The clinical and laboratory diagnostic features and the management of HSE case-patients were also described. HSE patients were divided into poor vs. favourable outcome groups. A favourable outcome was defined by the absence of major impairment on discharge or a Rankin test score <3 one month after discharge. A poor outcome was defined by death, discharge to a long-term facility, major impairment or a Rankin test score ⩾3. Both groups were compared with regard to the received treatment in univariate and multivariate analyses. The univariate relationship between outcome and ACV dose or duration were measured using χ2 test for categorical data or Student's t test for continuous variables. Because it had been previously demonstrated that outcome was associated with the delay between onset of symptoms and the start of ACV treatment [Reference Raschilas7], this variable was added to the logistic regression model, as well as age and the presence of comorbidities. The univariate and multivariate analyses were performed using Stata v. 11 (Stata Corporation, USA).
The decision criteria for continuing or discontinuing the ACV treatment were described for all case-patients enrolled in the study, with or without HSE, with reference to the timeliness of LP and to the results of HSV DNA detection in CSF samples. A positive HSV PCR in CSF was considered to be reliable without considering the timing of the LP. Assuming the HSV DNA detection by PCR in CSF samples was fully validated at each run, and good laboratory practice was observed for all patients and samples, a negative PCR was considered questionable if the CSF sample was obtained before day 4 or after day 10 of onset of neurological symptoms. A negative PCR was considered reliable if the CSF sample had been obtained between days 4 and 10 of neurological signs.
Informed consent was obtained from all patients or from their parents for children aged <18 years. According to French regulations, written informed consent could be obtained from the relative responsible for legal matters, or from the attending physician for patients unable to give consent due to the severity of their illness. However, this consent had to be confirmed by the patient once able to do so. The study was approved by the ethics committee of Grenoble (no. 172003).
RESULTS
Demography
Fifty-five (22%) of the 253 case-patients enrolled in the study had a diagnosis of HSE, 76 (30%) had an alternative diagnosis and the aetiology was unknown in 122 (48%).
All HSE patients but one were adult (median age 58 years, range 1 month to 85 years), and 31 (56%) were male. Seventeen (31%) reported comorbidities: four had cancer, one systemic lupus erythematosus, one congestive cardiac failure, three bipolar disorders and one senile dementia.
Clinical features (Table 1)
Twenty-six HSE patients (47%) were hospitalized on the first day of neurological symptoms, and four patients were hospitalized ⩾5 days after symptom onset. On admission, 48 (87%) presented with fever (range 38·5–40·4°C), all had altered mental status and nine (16%) required mechanical ventilation. On day 5 after admission, 30/54 (56%) surviving HSE patients still had fever, 41 (76%) still had altered mental status and nine (17%) required mechanical ventilation. All HSE patients had a positive HSV PCR on their first CSF sample: 42 (76%) were infected with HSV type 1, three (5%) with HSV type 2 and 10 (18%) with HSV of undetermined type.
Table 1. Clinical features of HSE patients on admission and on day 5 of hospitalization, France 2007
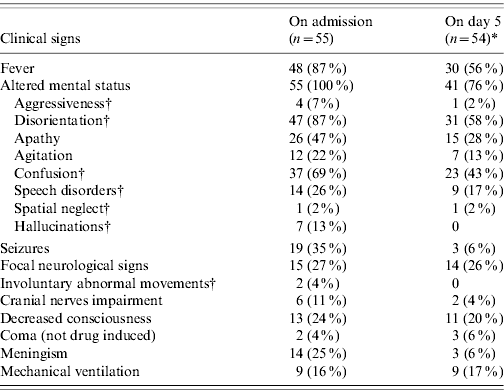
* A HSE patient died between admission and day 5.
† The percentage for these symptoms is calculated in 54 adult HSE patients in the first column and 53 adult HSE patients in the second column, as they are not relevant for the 1-month old child. This young patient had a recent history of rhinitis and cough, and he presented with high fever, decreased response to stimuli followed by seizures and respiratory distress.
Imaging
Fifty HSE patients (91%) had a CT scan on admission after a median duration of neurological symptoms of 1 day (range 0–10 days), and 40 (71%) had an MRI after a median duration of 3 days (range 0–10 days).
Altogether, 24 (48%) HSE patients had an abnormal CT scan and 38 (95%) had an abnormal MRI. Thirty-six HSE patients had both a MRI and a CT scan, 15 (42%) of which were concordant. MRI revealed lesions in 21 HSE patients with normal CT scan results. Lesions were most frequently temporal (n=48, 87%) or frontal (n=16, 29%) (Table 2). Fifteen (27%) HSE patients had lesions involving several lobes of the brain, 14 of which had a temporal lesion.
Table 2. Distribution of brain lesions of HSE patients on MRI and CT scan, France 2007
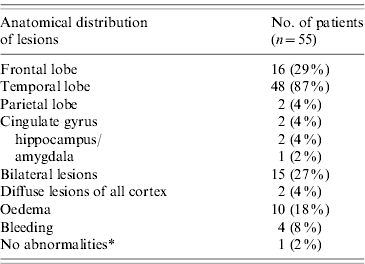
Most patients had more than one lesion.
* This patient had a normal CT scan and MRI, but temporal abnormal activity on EEG.
A 71 year-old HSE patient had a normal CT scan and a normal MRI. He presented with high fever (40°C) and a mild disease that improved quickly. He was discharged on day 17.
Outcome
Three (5%) HSE patients died:
• An 80-year-old man with malignant haemopathy and chronic respiratory failure died on day 4 of hospitalization with septic shock
• A 76-year-old man with ongoing prostatic cancer was found unconscious at home 2 days after failing to return phone calls. Awake but with decreased consciousness, he had temporal hyperintensity in T2-FLAIR MRI on admission. On day 4, he presented with complete absence of brainstem reflexes and cardiac arrest. He was resuscitated but died on day 11.
• A 49-year-old man with a history of cavernous sinus epidermoid carcinoma. He was admitted on the first day of neurological signs and was in a coma on day 5. He died on day 91 from a nosocomial infection.
Twenty-four (44%) patients were discharged home; 20 (36%) went to a convalescence facility; three (5%) to a long-term facility; two (4%) to a nursing home; one was admitted to another ward for management of a pre-existing bipolar disorder; one was transferred to a hospital in his native country; and one was lost to follow-up.
Of the 52 surviving patients, four (8%) were considered to have made a good recovery and 48 (92%) were discharged with persistent neurological symptoms: memory impairment (n=31); speech disorders (n=18); cognitive impairment (n=15); disorientation (n=11); focal neurological signs (n=8); dysexecutive syndrome (n=3); disinhibition (n=2); perseveration (n=2); and hallucinations or aggressiveness or delusion (n=1 for each). Altogether, 26 (47%) HSE patients had a poor outcome and 29 (53%) had a favourable outcome. The outcome was not significantly associated with the dosage or the duration of ACV treatment, with or without adjustment for comorbidities, age and time between neurological onset and first LP/start of ACV (see below) (Table 3).
Table 3. Univariate and multivariate association between age, acyclovir (ACV) treatment and comorbidities with the outcome of adult HSE patients, France 2007
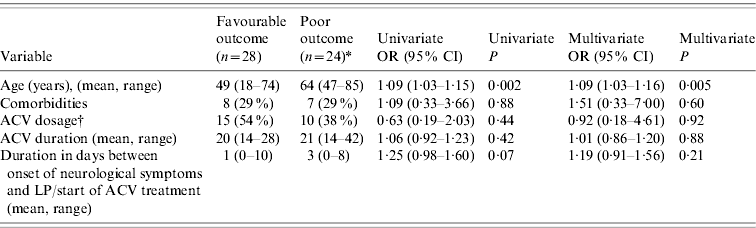
* Two patients who died before the end of treatment (at day 4 and day 11, respectively) are excluded from this analysis.
† 10 mg/kg t.i.d. vs. 15 mg/kg t.i.d. The number of cases and percentages are given for the 10 mg/kg t.i.d. treatment.
Management of ACV
All 253 encephalitis case-patients had a HSV PCR on admission. In all patients, ACV was initiated on the same day as the LP on which the HSV PCR was performed. The median time from onset of neurological signs to HSV PCR and ACV treatment was 2 days (range 0–10 days) in HSE patients and 1 day (range 0–16 days) in non-HSE case-patients.
Ten (18%) HSE patients were given a 2-week course of ACV, 42 (76%) a 3-week course of ACV and one received two courses of 21 days each due to a clinical relapse. Two patients died during treatment and therefore received incomplete courses of 4 and 11 days.
Information on the dosage of ACV prescribed was available for 45 (83%) adult HSE patients: 25 (53%) received 10 mg/kg t.i.d. and 22 (47%) received 15 mg/kg t.i.d. The 1-month-old HSE patient received 20 mg/kg t.i.d.
Of the non-HSE encephalitis case-patients, 20 had varicella-zoster virus (VZV) encephalitis and received ACV treatment according to experts' and manufacturers' recommendations.
Altogether, 71 non-HSV non-VZV encephalitis case-patients received a treatment that did not fit with the recommendations (Fig. 1). Of these:
• Thirty-one (44%) received a full course of ACV (2 or 3 weeks) despite negative PCR results from a CSF sample taken between days 4 and 10 after onset of neurological signs.
• Seven (10%) encephalitis case-patients with an alternative diagnosis received ACV after the assessment of the aetiological diagnosis.
No renal failure occurred in the 31 encephalitis patients with a full course of ACV despite reliable negative results or in the seven encephalitis patients with an alternative diagnosis.
• Thirty-three (46%) received ACV courses of shorter duration than recommended in the international literature (<2 weeks) despite HSV PCR being performed on CSF that might have given a false-negative result due to the LP being performed at an early time-point (Fig. 1). None of these 33 patients died during their hospital stay.

Fig. 1. Acyclovir (ACV) management in encephalitis patients with HSE and non-HSV varicella-zoster virus (VZV) encephalitis. * None received the 10-day ACV course recommended in the product licence. † Of these, five patients received exactly 10 days of ACV treatment. ‡ Of these, two patients received a 10-day course of ACV that might have been compatible with the product licence.
DISCUSSION
Our study differed from previous reports, due to its prospective design and the inclusion of HSE patients over a short 12-month time period. Consequently comparisons drawn between patients were less susceptible to variations in management and the performance of diagnostic tools due this short inclusion period. However, we can not be sure that our results are representative of the usual management of HSE patients and ACV in France, as the awareness and practice of the investigators might have been influenced by the study being performed.
Our case definition referred to HSV DNA detection by PCR in CSF only. Although this test has proven high sensitivity and specificity, these are not 100% and some cases might still be misclassified. However, we are confident that we did not misclassify false-positive cases considering the convergence of clinical signs and imaging results with HSV PCR results. It has been hypothesized that the use of ACV might be responsible for the elevation of the HSV DNA in CSF after a few days of treatment [Reference Roos13]. In our study, all cases were diagnosed by a positive PCR performed on the CSF taken on admission. The timeliness of the LP makes it highly improbable that ACV might have influenced the result of the PCR for both HSE and non-HSE patients. HSE patients with an initial normal CSF might not have fitted the case definition and therefore not been enrolled if later CSF samples had not been collected and analysed. However, undiagnosed HSE patients might have experienced severe complications leading to a late diagnosis (especially extensive necrosis of the cerebral cortex). However, the case definition was designed for this study and might be too specific for the management of patients in everyday life. For example, fever might be absent due to the self-prescription of antipyretic drugs. By contrast, no imaging criteria were used in our study because the imaging devices were not comparable in all hospitals enrolling patients (MRI or scan, more or less recent devices). However, the individual management of encephalitis is likely to take into account the results of CT scan or MRI in suspected HSE.
Some cases enrolled in our study with no aetiological diagnosis could have been HSE patients with false-negative HSV PCR results (inappropriate timing of CSF sampling). Some patients were prescribed a full course of ACV and therefore a possible false-negative result may have had few or no consequences. On the contrary, 33 encephalitis patients received a shorter course of ACV than recommended despite the questionable reliability of the negative PCR. If these 33 patients had had an undiagnosed HSE, and assuming that the CFR observed in the absence of treatment (70% [Reference Whitley6]) is the same with non-sufficient ACV treatment, an average of 23 deaths would have been expected. None died or were discharged with major sequelae, making it unlikely that they were undiagnosed HSE patients. Moreover, the complete set of data was examined for each enrolled patient at the end of the inclusion period by a panel of experts on the Steering Committee. Of the patients of unknown aetiology, none had clinical signs and imaging consistent with HSE. However, because unusual presentations are possible although rare, performing a HSV PCR on a later, second sample could still be recommended if the timing of the first LP might lead to an unreliable result.
In our study, most courses of ACV were longer than the 10 days recommended in the marketing recommendations. Clinical trials have demonstrated ACV to have a better efficacy than vidarabine [Reference Sköldenberg14, Reference Whitley15], but no trial has been designed to assess the dosage of ACV or the duration of treatment. The professional consensus is that a prescription shorter than 14 days increases the risk of relapse, and that ACV should be administered for 14–21 days [Reference Steiner, Kennedy and Pachner9]. HSE relapses have been described in neonates and children [Reference Whitley, Sheld, Whitley and Marra1], but only a few cases were reported in adults treated with ACV (Table 4). These publications, although valuable, are not comparable to a clinical trial. Moreover, they do not mention all factors considered to influence the outcome, for example coma on admission or the time between neurological onset and the beginning of the ACV course. Finally, only two of these papers reported studying HSE relapses [Reference Kimura21, Reference Sköldenberg25].
Table 4. HSE relapses reported in adults (>15 years) following acyclovir (ACV) treatment in the literature
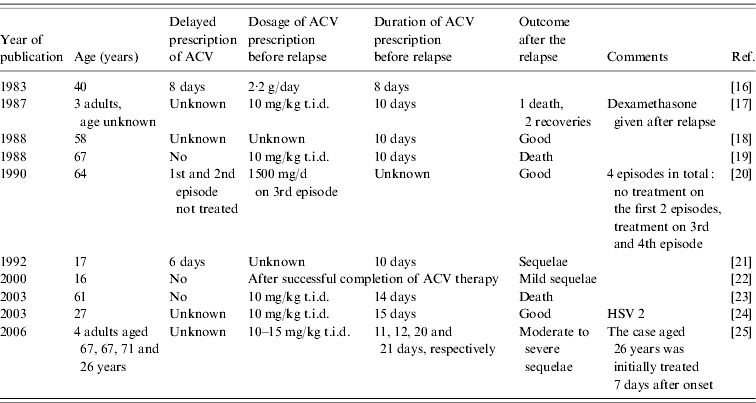
To avoid relapses, some authors recommend performing a final HSV PCR on CSF at the end of the treatment period [Reference Baringer26]. The practice of performing an uncomfortable LP at the end of therapy in patients with clinical improvement is questionable, and no evaluation of the interpretation of a final PCR has been undertaken. Moreover, besides persistent viral replication, immune or inflammatory mechanisms have been identified as a cause of relapse in HSE, with no evidence of any effect of ACV on these non-infectious processes [Reference Sköldenberg25].
In our study, one adult HSE patient had a relapse of neurological signs despite an initial 21-day treatment. This patient was treated for another 21 days with no drug-related complications, then discharged to a convalescent facility. Currently, this patient still has a major neurological impairment and has been unable to resume either professional activity or a normal social life.
The influence of the delay before ACV is given on the patient's prognosis has been demonstrated previously [Reference Raschilas7, Reference Martinez-Torres11, Reference Bell, Suckling and Rothburn27]. In our study, this delay as well as the duration of treatment and dosage of ACV were not associated with the outcome, probably because of lack of power. The duration of treatment might also be influenced by the evolution of the patient's condition during treatment. Our study was not designed to assess the efficacy of ACV treatment, and only a clinical trial could answer such questions. Besides the management of patients and their treatment, it has been suggested that the viral genotype might influence the severity of the disease and the outcome.
The clinical features of our HSE patients were consistent with those of former case-series, especially concerning MRI results [Reference Taylor, Lee and Jackson28]. Combining MRI and CT scans demonstrated lesions in 54/55 (98%) HSE patients, highlighting the sensitivity of these methods.
The CFR was lower than reported in the literature. This might be due to early treatment with ACV being widely used in French hospitals. The recommendations for early treatment with ACV, before the HSV PCR result, have been observed in France and abroad [Reference Raschilas7, Reference Cinque29, Reference Stahl30]. However, previous French studies on HSE demonstrated higher mortality [Reference Raschilas7] but the patients were mainly admitted to intensive-care units (ICUs) and those series were restrospective, involving patients whom had been diagnosed in 1991.
Another explanation for the low CFR in our study might be the enrolment of patients in first-line hospitals, as well as university hospitals. Patients enrolled in first-line hospitals and not transferred to second- or third-line hospitals might have had a less severe illness. Clinicians should be aware of such unusually mild presentations, as they also require close medical attention in order to avoid disease progression. Moreover, previous papers might have enrolled patients mainly hospitalized in ICUs, or some investigators might have included more severe forms of HSE as the management of mild cases was easier. In our study, two of the three HSE patients who died had ongoing cancer, making it difficult to fully attribute their death to HSE. Most publications report an average CFR of 14–20% after various delays from 1 month to 1 year [Reference Whitley, Sheld, Whitley and Marra1, Reference Raschilas7, Reference Tunkel8, Reference Martinez-Torres11]. In our study, death was reported only during the hospitalization for the acute episode. Some patients might have died since they were discharged from hospital, from causes directly or indirectly linked with their encephalitis. Consequently the CFR observed in our study might be underestimated.
However, HSE can have serious long-term consequences in surviving patients for whom resumption of their former life is sometimes impossible due to psychological reasons [Reference Hokkanen and Launes31]. Most of our studied HSE patients were not considered cured on discharge. Memory impairment and dysexecutive syndrome were the most frequent persisting symptoms. These sequelae require long-term follow-up, sometimes but not always with measurable improvement.
CONCLUSION
We identified 55 HSE patients in France during a 1-year period, with a very low CFR despite clinical presentations ranging from mild disease to severe features requiring hospitalization in ICUs. Our findings suggested good management of confirmed HSE patients in France. ACV should always be started as soon as possible in patients with encephalitis, even if the presentation is not typical of HSE. Because the reliability of a HSV PCR result on a CSF sample collected early on admission to hospital is questionable, and a LP is not a life-threatening procedure, a PCR should always be performed after 4 days of symptoms. The treatment has to be administered for at least 2 weeks at a sufficient dosage. However, after the acute episode, special attention should be given to patients and the relatives caring for them who are discharged with subacute persisting symptoms that no longer require hospitalization.
ACKNOWLEDGEMENTS
Institut de veille sanitaire (French Institute for Public Health Surveillance), Saint Maurice, France promoted the study, funded the salary of A.M. and of two data collection personnel. GlaxoSmithKline, Roche, bioMérieux and SPILF (French Infectious Diseases Society) funded the setting up and maintenance of a biobank of samples taken from patients. SPILF organized the network of investigators.
APPENDIX
Steering Committee
Cecile Bébéar (Bordeaux), Cecile Brouard (Saint-Maurice), Thomas De Broucker (Saint-Denis), Eric Cua (Nice), Henri Dabernat (Toulouse), Daniel Floret (Lyon), Benoit Guéry (Lille), Marc Lecuit (Paris), Daniel Lévy-Bruhl (Saint Maurice), Bruno Lina (Lyon), Olivier Lortholary (Paris), Alexandra Mailles (Saint-Maurice), Jean-Claude Manuguerra (Paris), Patrice Morand (Grenoble), Christian Michelet (Rennes), Hélène Peigue-Lafeuille (Clermont-Ferrand) Bruno Pozzetto (Saint-Etienne), Jean-Paul Stahl (Grenoble), Veronique Vaillant (Saint-Maurice), Yazdan Yazdanpanah (Tourcoing), Herve Zeller (Lyon).
Investigators
Philippe Abboud (Rouen), Chakib Alloui (Paris), Christine Archimbaud (Clermont-Ferrand), Bruno Barroso (Pau), Louis Bernard (Garches), Pascal Beuret (Roanne), Geneviève Billaud (Lyon), Thierry Blanc (Rouen), Michèle Bonnard-Gougeon (Clermont-Ferrand), David Boutolleau (Paris), Cédric Bretonnière (Nantes), Céline Bressollette-Bodin (Nantes), Fabrice Bruneel (Versailles), Marielle Buisson (Dijon), Anne Caramella (Nice), Bernard Castan (Auch), Isabelle Cattaneo (Bry sur Marne), Charles Cazanave (Bordeaux), Stéphane Chabrier (Saint-Etienne), Marie-Laure Chadenat (Versailles), Martine Chambon (Clermont-Ferrand), Pascal Chavanet (Dijon), Mondher Chouchane (Dijon), Pierre Clavelou (Clermont-Ferrand), Pierre Courant (Avignon), Eric Cua (Nice), Fabienne de Brabant (Montélimar), Arnaud De La Blanchardière (Caen), Geoffroy De La Gastine (Caen), Henri De Montclos (Bourg-en-Bresse), Eric Denes (Limoges), Philippe Desprez (Strasbourg), Anny Dewilde (Lille), Aurelien Dinh (Garches), François Durand (Saint-Etienne), Guillaume Emeriaud (Grenoble), Olivier Epaulard (Grenoble), Giovanni Favaretto (Avranche), Anna Ferrier (Clermont-Ferrand), Vincent Foulongne (Montpellier), François Fourrier (Lille), Véronique Gaday (Pontoise), Jacques Gaillat (Annecy), Serge Gallet (Montluçon), Marie-Ange Gau (Montpellier), Nicole Gazuy (Clermont-Ferrand), Hugues Georges (Tourcoing), Stéphanie Gouarin (Caen), Pascale Goubin (Caen), Alain Goudeau (Tours), Joel Gozlan (Paris), Philippe Granier (Bourg-en-Bresse), Michèle Grappin (Dijon), Isabelle Gueit (Rouen), Amélie Guihot (Paris), Christine Guillermet (Besançon), Christelle Guillet-Caruba (Paris), Yves Guimard (Bourges), Yves Hansmann (Strasbourg), Cécile Henquell (Clermont-Ferrand), Jean-Louis Herrmann (Garches), Jérome Honnorat (Lyon), Nadhira Houhou (Paris), Benoit Jaulhac (Strasbourg), Olivier Join-Lambert (Paris), Manoelle Kossorotoff (Paris), Emmanuelle Laudrault (Montélimar), Frédéric Laurent (Lyon), Jean-Jacques Laurichesse (Paris), Sylvain Lavoue (Rennes), Leila Lazaro (Bayonne), Stephane Legriel (Versailles), Olivier Lesens (Clermont-Ferrand), Gérard Level (Verdun), Muriel Mace (Orléans), Bénédicte Maisonneuve (Montluçon), Alain Makinson (Montpellier), Hélène Marchandin (Montpellier), Stéphanie Marignier (Lyon), Laurent Martinez-Almoyna (Saint-Denis), Patrick Marthelet (Montélimar), Martin Martinot (Colmar), Bruno Massenavette (Lyon), Laurence Maulin (Aix-en-Provence), Audrey Mirand (Clermont-Ferrand), Benoit Misset (Paris), Catherine Neuwirth (Dijon), Florence Nicot (Toulouse), Jérome Pacanowski (Paris), Jean-Bernard Palcoux (Clermont-Ferrand), Patricia Pavese (Grenoble), Thomas Perpoint (Lyon), Martine Pestel–Caron (Rouen), Robin Pouyau (Lyon), Thierry Prazuck (Orléans), Virginie Prendki (Paris), Christophe Rapp (Saint-Mandé), Christel Regagnon (Clermont-Ferrand), Laurent Renie (Aix-en-Provence), Florence Ribadeau-Dumas (Paris), Matthieu Rigal (Auch), Nathalie Roch (Grenoble), Olivier Rogeaux (Chambéry), Sylvie Rogez (Limoges), Charles Santre (Annecy), Anne Signori-Schmuck (Grenoble), Fabrice Simon (Marseille), Abdelilah Taimi (Roanne), Jérome Tayoro (Le Mans), Daniel Terral (Clermont-Ferrand), Audrey Therby (Versailles), Francis Vuillemet (Colmar).
DECLARATION OF INTEREST
None.







