Introduction
Hepatitis B is a disease caused by the hepatitis B virus (HBV), which is mainly associated with liver inflammatory lesions and can result in multiple organ damage. The World Health Organization (WHO) has estimated that 296 million people were living with chronic hepatitis B infection in 2019, with 1.5 million new infections each year. In addition, the number of related deaths in 2019 was 820,000, mainly caused by HBV-induced liver cirrhosis and liver cancer [1]. Nowadays, there is no method that can cure chronic hepatitis B infection completely, but the hepatitis B vaccine (HepB) is the most effective method to prevent HBV infection [2]. In 1992, HepB was included in the planned immunization management in China, and it was further included in the Childhood Immunization Programme to implement free vaccination for newly born children in 2002, with efforts being made to improve the whole-course vaccination rate of children and the timely vaccination rate for the HepB birth dose (HepB-BD).
At present, numerous studies have focussed on epidemiological investigation of the positivity rate of hepatitis B surface antibody (HBsAb) in people over 1 year old [Reference Fuzhen3–Reference Zanella9]. However, the antibody level, assessed monthly or at more frequent intervals after each of the three doses, has not been previously reported. To elucidate the level of antibody formation at various times after injection, the current study used the detection data of HBsAb and hepatitis B surface antigen (HBsAg) of hospitalized children to analyze the HBsAb level after immunization, with reference to their vaccination history.
Methods
Subjects
Retrospective collection of serological data from children hospitalized in Anhui Provincial Children’s Hospital from January to May 2019, a total of 20,946 hospitalized children aged 0–14 years were tested for HBsAb and HBsAg in Anhui Provincial Children’s Hospital, of which 7,250 hospitalized children in the surgical system (general surgery, ophthalmology, otolaryngology, orthopaedic surgery, urology, and stomatology) and neonatal department were selected, and their vaccination information was collected from the Anhui immunization planning information management system. After excluding 43 children who received the Chinese hamster ovary cell recombinant HepB, 442 who were unvaccinated against HepB, and 29 who were vaccinated with more than 3 doses of HepB, these 6,736 children vaccinated Yeast cell recombinant HepB (not the same brand) were divided into one-dose, two-dose, or three-dose groups according to their hepatitis B vaccination status (See Figure 1).

Figure 1. Flow diagram of selection and classification of subjects. Dc: Date of blood collection; Db: Date of birth; D1: Date of the first dose of HepB; D2: Date of the second dose of HepB; D3: Date of the third dose of HepB; D4: Date of the fourth dose of HepB. 1 dose HepB: The group only vaccinated one-dose HepB; 2 doses HepB: Completed two doses of HepB vaccination; 3 doses HepB: Completed three doses of HepB vaccination.
Sample collection and detection
Children’s fasting peripheral venous blood samples of 2–3 mL were drawn, centrifuged at 4000 rpm for 15 min, and then the supernatant was collected. HBsAg and HBsAb markers were detected in serum using the CL-2000i automated chemiluminescent immunoassay analyzer according to the instructions for the HBsAg and HBsAb detection kits.
Reagents and instruments
A CL-2000i automatic chemiluminescent immunoassay analyzer, HBsAg and HBsAb detection kits, and HBsAg and HBsAb quality control samples were all purchased from China Mindray Medical Co., Ltd.
Result interpretation standard
Referring to the manuals of these detection kits, an HBsAg concentration of ≥0.08 IU/mL was regarded as positive; otherwise, it was negative. An HBsAb concentration of ≥10.0 mIU/mL was regarded as positive; otherwise, it was negative. The results of both the HBsAg and HBsAb quality control samples were within the quality control range.
Statistical analysis
Excel 2016 and SPSS 13.0 software were used for statistical analysis. The HBsAb concentration is described by the geometric mean concentration (GMC) and its 95% confidence intervals (CI). HBsAb and HBsAg levels were analyzed within 1 year after one, two, and three doses of HepB vaccination, respectively. Moreover, the antibody levels at different times within 14 years after vaccination in children who were fully vaccinated were also analyzed.
Results
Demographic characteristics of the subjects
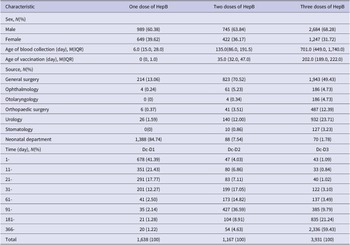
A total of 6,736 subjects were included in this study, of which 4,418 were male and 2,318 were female. The overall positivity rate of HBsAb and HbsAg were 76.02% (5,121/6736) and 0.06% (4/6736). Most of the children were from general surgery, urology, and neonatal department. The HepB vaccination procedure for children is 10 μg three doses of yeast cell recombinant HepB at zero, one, and six months. At the time of blood collection, 1,638, 1,167, and 3,931 children had received one dose, two doses, and a full dose of HepB, respectively (See Table 1).
Table 1. General characteristics and vaccination history of the subjects
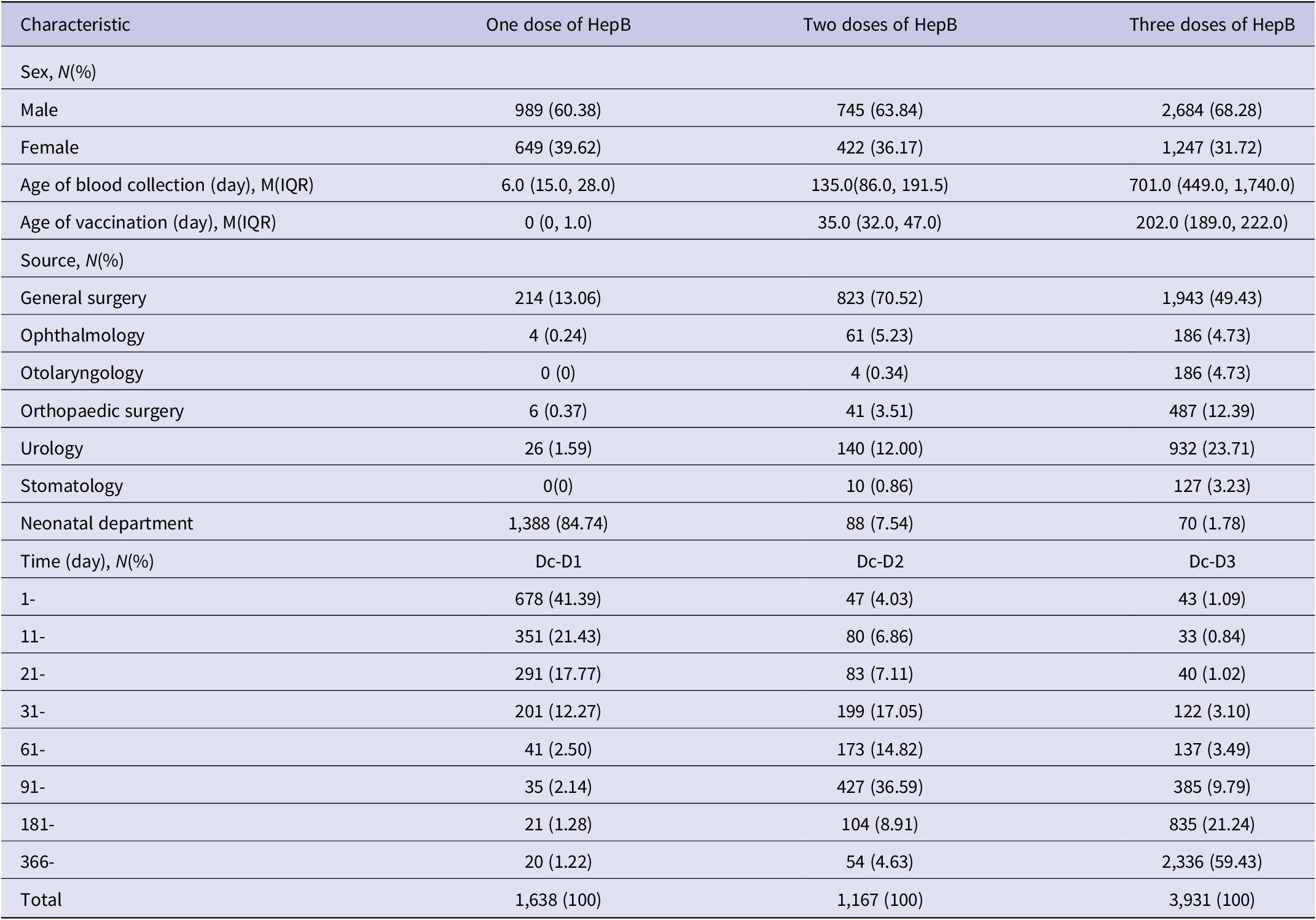
Abbreviations: Dc, date of blood collection; D1, date of the first dose of HepB; D2, date of the second dose of HepB; D3: Date of the third dose of HepB.
HBsAb levels in the one-dose HepB group
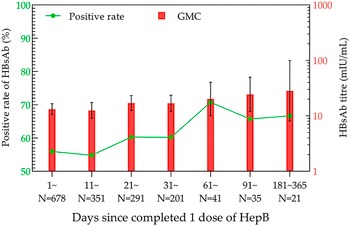
Within one year after the first HepB vaccination, the positivity rate volatility rose, with the highest rate of 70.73% in the 61–90-day group. Meanwhile, GMC showed a gentle increasing trend, and the highest GMC was 28.24 mIU/mL (95% CI: 8.07–98.76 mIU/mL) in the 181–365-day group (Figure 2).

Figure 2. HBsAb levels in the one-dose HepB group within one year.
HBsAb levels in the two-dose HepB group
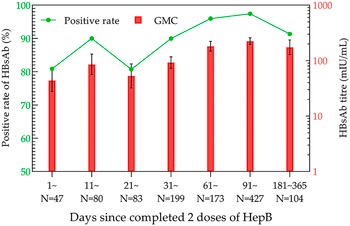
Although there were some variations in the data for the 21–30- and 181–365-day groups, there was an increasing trend in the overall positivity rates and GMC after the second dose of HepB. The positivity rate and GMC of HBsAb were the highest in the 91–180-day group, which were 97.42% and 224.11 mIU/mL (95% CI: 195.50–256.90 mIU/mL), respectively (Figure 3).

Figure 3. HBsAb levels in the two-dose HepB group within one year.
HBsAb levels in the three-dose HepB group
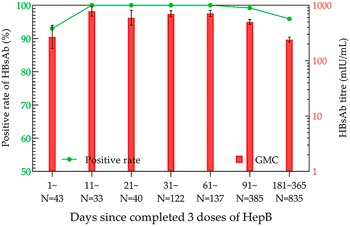
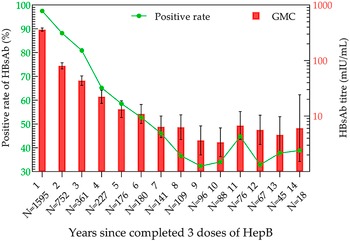
After the completion of full immunization, the positivity rate and GMC of HBsAb were the highest in the 11–20-day group, which were 100% and 784.15 mIU/mL (95% CI: 640.49–960.03 mIU/mL), respectively (Figure 4). Then, the analysis of antibody levels in 3931 children within 14 years after three doses of immunization demonstrated that the positivity rate and GMC of HBsAb decreased with time. The lowest positivity rate (32.29%) and GMC (3.37 mIU/mL; 95% CI: 1.85–6.13 mIU/mL) were observed at years 9 and 10 post-vaccination, respectively. After that, the positivity rate and GMC were maintained at 30%–45% and 3–7 mIU/mL until the 14th year, respectively (Figure 5).

Figure 4. HBsAb levels in the three-dose HepB group within one year.

Figure 5. HBsAb levels during the 14 years in the three-dose HepB group.
HBsAb level within one year after one, two, and three doses of HepB
The positivity rates and GMC of HBsAb were higher with increasing doses of HepB, and their time to peak was shorter with each additional dose. The positivity rate and GMC of HBsAb remained above 90% and 100 mIU/mL, respectively, within one year after the completion of three vaccination doses.
Discussion
The pattern of antibody formation after vaccine immunization has been described in many papers and textbooks, but it was difficult to observe the precise antibody level after one, two, and three doses by continuous blood collection among young children, especially within one year of birth. This was partly due to the difficulty of collecting blood multiple times from children in the younger age groups, the obstacle of obtaining support from children and their parents, and the high cost of organizing investigations and blood collection. Therefore, firstly, most studies collected serum at 1–3 time points after immunization [Reference Ballesteros-Trujillo10–Reference Milne12]. Secondly, these time points cover a wide span of age groups, with few data available up to one year after vaccination [Reference Yuen13]. Thirdly, a few studies collected blood samples in certain months of the 1-year-old age group, but the sample size per group was relatively small, with a maximum of 30 samples per group in the study by Jilg et al. [Reference Jilg, Schmidt and Deinhardt14]. Therefore, previous descriptions of antibody levels after three doses of HepB vaccination were not detailed and adequate. Regarding these, the current study used the available detection data of HBsAb and hepatitis B surface antigen (HBsAg) in hospitalized children (including a large number up to one year after vaccination) to analyze the HBsAb level after immunization combined with their vaccination history. With the data now available, our study aims to evaluate the hepatitis B antibody level after 1, 2, and 3 doses of vaccination. This will serve as a foundation for a subsequent investigation into the dynamic evolution of antibodies.
The results of the current study demonstrated that the highest positivity rates of HBsAb appeared at 61–90 days after HepB-BD immunization (70.73%), 91–120 days after the second dose of HepB immunization (97.42%), and 11–20 days after the third injection of HepB (100%), respectively. Correspondingly, the highest GMC values after each dose of HepB vaccination showed at 181–365 days (28.24 mIU/mL; 95% CI: 8.07–98.76 mIU/mL), 91–180 days (224.11 mIU/mL, 95% CI: 195.50–256.90 mIU/mL), and 11–20 days (784.15 mIU/mL; 95% CI: 640.49–960.03 mIU/mL). Both the positivity rate and GMC increased sequentially with the immunization dose, reaching their peaks earlier after the third dose than after the first two doses.
For the evaluation of immunization efficacy after HepB vaccination, the WHO position paper (2017 version) [15] stated that a positive HBsAb (≥10 mIU/mL) in the blood, collected one to two months after the completion of full HepB immunization (one to three months after immunization was propounded in Vaccines [Reference Plotkin, Orenstein and Offit16]), indicated long-term effective protection against HBV. Many studies have reported that approximately 5% of the vaccinees fail to produce effective protective antibodies (HBsAb <10 mIU/mL) after HepB vaccination. In contrast, all 332 cases tested positive for HBsAb in blood collected 11–90 days after three full doses of immunization in our study, which was higher than that reported in previous studies. These studies could be divided into the following categories. In the first category, specimens were collected more than three months after immunization [Reference Hui, Fu-zhen and Yuan-sheng17–Reference Safadi19], or even two years after immunization [Reference Nani20]. When our study followed their blood collection time, the results were also similar. The second category was that the study population was a high-risk group for hepatitis B, such as close relatives of HBV carriers [Reference Yuen13] and newborns of HBsAg-positive mothers [Reference Safadi19, Reference Zou21]. Then, the third included studies in which the subjects were based on or included adolescent populations [Reference Catania, Di Ciommo and Concato22, Reference Stevens23]. Previous studies have illustrated the fact that the peak of HBsAb formation in older children or teenagers is slightly later than that in neonates [Reference Honorati24, Reference Hwang25], thus being more likely to lead to undetectable HBsAb. The last category included studies aiming at different immunization procedures. Jilg et al. [Reference Jilg, Schmidt and Deinhardt14], Goldfarb et al. [Reference Goldfarb26], Sintusek et al. [Reference Sintusek27], and Greenberg et al. [Reference Greenberg28] studied the difference in the HBsAb positivity rate according to varying vaccination times (0, 1, 6; 0, 1, 12; 2, 4, 6 months) and varying dosages (5, 10, and 20 μg immunization). Taking the above factors into account, it could be reasonably explained that the positivity rate of HBsAb in the current study was higher than that in previous studies. Therefore, we prefer to believe that the proportion of HBsAb non-response should be less than 5% after full immunization with HepB in a normal population, provided that the appropriate time for blood collection is chosen.
For the subjects of this study, it was more appropriate to collect blood 11–90 days after the three doses of HepB immunization to detect HBsAb. The present timeline was more precise than the one- to two- and one- to three-month time frames indicated in the WHO position paper (2017 version) and Vaccines.
In terms of antibody duration, the positivity rate and GMC of HBsAb could be maintained at above 90% and 100 mIU/mL after three doses of HepB immunization, respectively, within one year. The positivity rate and GMC fell below 50% and 10 mIU/mL by seven years post-vaccination and reached the lowest (32.29% and 3.37 mIU/mL) by years 9 and 10. The highest and lowest positivity rates of HBsAb in the 2014 Anhui Province Hepatitis B Serological Survey were also found in the one-year-old group (91.89%) and the 9–10-year-old group (39.13%) [Reference Ying, Jihai and Yu29]. The two results were consistent. However, the 2014 survey only conducted qualitative testing, so the GMC could not be calculated. Furthermore, the study subjects were between 1 and 29 years old, and children younger than one year old were not included.
Although this was the first study to specifically explore the antibody levels 0–14 years after HepB immunization in hospitalized children, there were some limitations to this work. First, the data are not longitudinal, and there is individual heterogeneity in antibody levels after each of the three doses; therefore, they can only be used to provide a reference for the dynamic change of antibody levels after immunization. Finally, there was selection bias when compared to the normal population since the subjects of this study were inpatients (unhealthy children). In the selection of the study population, we excluded cases from the department that were prone to hepatitis B patient visits; therefore, the number of hepatitis B virus carriers and infections with no or low response to the vaccine was reduced. As a result, the HBsAg positivity rate of the included subjects was closer to that of the normal population. The results of this study showed that the HBsAg positivity rate was 0.06% (4/6736), consistent with the decreasing trend of HBsAg in several serological surveys of hepatitis B in Anhui Province (the positivity rate of HBsAg in children aged 1–14 years in 2006 was 1.49% (20/1340) [Reference Yu30]and in 2014 was 0% (0/851) [Reference Ying, Jihai and Yu29]. And among children <15 years of age, HBsAg prevalence declined from 10.5% to 0.8% in China, during 1992–2014 [Reference Cui31]), expecting that the results of the analysis would have some reference values for the normal population, although this is the biggest limitation of this study.
Data availability statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to them containing private information about individuals.
Author contribution
Clinical testing: Ll.X., and Jy.H., and Lj.Z.; Conceptualization: Y.C., Jh.T., and Lj.Z.; Data collection, Zj.L., X.C., and Jl.H.; Formal analysis: Y.C. and K.X.; Funding acquisition: Y.C., Jh.T., and Lj.Z.; Methodology, Y.C. and Y.S.; Resources: Bb.W. and Xw.L.; Writing—original draft preparation: Y.C.; Writing—review and editing: Jh.T. and K.X. All authors read and agreed to the published version of the manuscript.
Financial support
This research was funded by the Chinese Foundation for Hepatitis Prevention and Control (grant number YGFK20190031).
Competing interest
The authors declare none.
Ethical standard
No blood specimens were taken specifically for this investigation because it relied on prior HBsAb and HBsAg test results from the hospital. Therefore, ethical review and approval were waived for this study.