INTRODUCTION
Throughout the developing world, diarrhoea is associated with high childhood morbidity and mortality, both directly and indirectly through proximal pathways involving malnutrition and immunodepression [Reference Gupta1]. The disease places an enormous burden on health systems, particularly in outbreak and epidemic contexts or during seasons of high transmission.
Seasonal fluctuations in diarrhoea are a well known and well documented phenomenon [Reference Dowell2–Reference Epstein5]. The effects of seasonal variations on diarrhoea transmission may operate at many different levels. Weather, for example, can affect human behaviour by restricting access to sanitation and encouraging open defecation or toileting elsewhere [Reference Lipsitch and Viboud6]. Water shortages may result in a curtailing of hygiene practices or the consumption of dirty water, while flooding can contaminate water supplies [Reference Ansari, Springthorpe and Sattar7]. Other weather variables such as temperature can affect dominant circulating pathogen species and serotypes or growth patterns [Reference McCormick, Alonso and Miller8]. Indeed seasonal variations affect a number of variables which dictate host, pathogen and environmental characteristics, and the extent of their interaction [Reference Jagai9, Reference Nesbitt10]. Rotavirus – a common diarrhoea-causing pathogen has been found to be more prominent in South Asia during the cooler, drier months [Reference Jagai9], while the seasonality of other pathogens including Vibrio cholerae and Escherichia coli have also been documented [Reference Zo11–Reference Paredes-Paredes13]. In contrast, little variation has been found in the seasonality of Shigella spp. infections [Reference Bowman, Flint and Pollari14].
A study conducted in Bangladesh in the late 1980s revealed two peaks of diarrhoeal disease incidence; one in September–October [Reference Begum15] composed predominantly of cholera and rotavirus infections, and a second peak in April–May composed of cholera and enterotoxogenic E. coli (ETEC) infections [Reference Begum15–Reference Albert20]. Rahman and colleagues showed significant correlation between increased incidence of rotavirus infection and water level or temperature [Reference Rahman21]. However, most of them were published more than a decade ago with few exceptions [Reference Begum15–Reference Rahman21] and did not determine the seasonality patterns following appropriate methodology and in different geographical locations without considering any urban–rural differentials.
The International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) maintains a diarrhoeal disease surveillance system [Reference Stoll16] in four different geographical locations; two urban diarrhoeal disease hospitals [Dhaka Hospital (DH) and Mirpur Treatment Centre (MTC)] in Dhaka and two rural hospitals (Matlab Hospital, Matlab (MH), Kumudini Women's Medical College and Hospital, Mirzapur (KH)]. These facilities highlight on the recent seasonal variations of common enteric pathogens causing diarrhoea in Bangladesh. This study aims to promote an understanding of the recent seasonal variations of four common infections (Shigella, rotavirus, V. cholerae, ETEC) in four different settings, and hospitalization patterns including presenting features of patient population and their urban–rural differentials.
METHODS
Bangladesh weather
Bangladesh is a low-lying country in South Asia situated between the foothills of the Himalayas and the Indian Ocean. The country is characterized by flat plains, dissected by numerous interconnected rivers, rendering the country vulnerable to both flooding and drought. The combination of topography with tropical monsoon weather makes Bangladesh a hotspot for diarrhoea. Bangladesh has three distinct seasons, the hot summer season from March to June; the main rainy season from June to September; and the cool winter period from October to February [22]. June is the transitional month of summer to monsoon with year to year variation. For this analysis, we considered June the summer peak.
Bangladesh healthcare system
Recent literature has revealed that 88% of caregivers of children aged <5 years in rural Mirzapur sought care outside the home for diarrhoeal diseases for their children; 50% from a pharmacy, and 22% from a hospital or health centre [Reference Das23]. The study reported poor maternal perception regarding the seriousness of diarrhoeal illnesses (74%) and the high cost of treatment (22%) as the primary reasons for not seeking care [Reference Das23]. However, other work has reported distance to the nearest healthcare facility as an important confounder [Reference Ferdous24]. Another study in Matlab revealed, being a female patient, absence of low-cost health services, and illnesses of relatively short duration were associated with self-care. Seeking medical attention from a healthcare facility was predicted by male sex, geographical location, higher socioeconomic status, and serious illness of longer duration [Reference Ahmed25]. Moreover, sex discrepancy and poor socioeconomic status were the likely confounders that were associated with seeking quality healthcare in urban Dhaka [Reference Najnin, Bennett and Luby26].
Study site
Dhaka Hospital (DH), icddr,b, Dhaka
The Dhaka Hospital of icddr,b is a specialized research, treatment and training facility for diarrhoeal diseases. The hospital has a catchment area consisting of the Dhaka metropolitan area and its suburbs although some patients travel from rural areas. The facility treats about 140 000 diarrhoeal cases each year. Since 1979, icddr,b has been running a clinic-based diarrhoeal disease surveillance system (DDSS), which systematically samples patients; 4% of all presenting in the triage area up to 1995, and 2% since 1996. The DDSS currently collects information on the clinical, epidemiological and demographic characteristics, feeding practices, particularly of infants and young children, and use of drug and fluid therapy at home of every 50th patient irrespective of age, sex, disease severity or socioeconomic status by administering a structured questionnaire. DDSS also monitors antimicrobial susceptibility of common bacterial pathogens. Tap water supplied by the city Corporation is the main source of water for the urban Dhaka population.
Mirpur Treatment Centre (MTC), Dhaka
This 60-bed urban hospital began operation in April 2009. More than 12 000 patients receive care in this urban facility annually, of which about 25% report with severe dehydration. Every 10th (10% sample) patient presenting to the facility is included in the surveillance system irrespective of age, sex, socioeconomic status, and disease severity. The source of water for drinking is also tap water.
Matlab Hospital (MH), Matlab
Since 1963, icddr,b has maintained a treatment facility in rural Matlab for treating diarrhoea patients. Matlab is a low-lying riverine area, located 57 km south-east of Dhaka. The facility has 120 beds, 70 of which are reserved for diarrhoea patients. The centre sees 15 000–20 000 diarrhoea patients reporting annually. All patients coming from the health and demographic surveillance system (HDSS) area are included in the hospital surveillance system. The HDSS maintains one of the richest, most comprehensive and longest running, longitudinal data resources in the developing world, and produces regular accurate demographic and health data for rural Matlab, Bangladesh. The HDSS at Matlab covers a population of about 225 000; providing data necessary to plan, conduct, and evaluate various types of public-health intervention researches. Tube-wells are the main source of drinking water in rural Matlab.
Kumudini Women's Medical College and Hospital (KH), Mirzapur
KH was established in 1938 in Mirzapur subdistrict; Tangail district, 60 km north-west of Dhaka. The hospital has 750 beds, of which 20–25 form a separate diarrhoea treatment unit. Nearly 1500 diarrhoea patients report to this unit annually. Since 2010, the diarrhoeal disease surveillance system has been in constant operation, obtaining data and faecal specimens from patients. All patients presenting from the demographic surveillance system (DSS) catchment area were included in the present study. According to a 2007 survey, the DSS includes a total of 58 300 households comprising a population of 238463. Almost all households have access to a tube well as a source of drinking water; around half have a hygienic sanitary toilet, and 60% have electricity.
Study time frame
Relevant data from all four sites for January 2010 to December 2012 were utilized to form the dataset for analysis.
Specimen collection and laboratory procedure
A single, fresh, whole stool specimen was collected from all enrolled patients, and submitted immediately to the laboratory in Dhaka (from DH and MTC) and Matlab (from MH). Faecal swabs from stool specimens of patients reporting to KH were placed in Cary–Blair medium in plastic screw-topped test tubes. All specimens were transported to the central laboratory in Dhaka in a cooler box, within 6 h of collection. All stool samples were routinely screened for common enteric pathogens including ETEC [Reference Qadri27], V. cholerae [28], Shigella spp. [28], and rotavirus [Reference Rahman21] applying standard laboratory methods.
Statistical analysis
Data entry and analyses were performed using SPSS for Windows v. 15·2 (SPSS Inc., USA). Age was categorized as: 0 to <2 years, 2 to <5 years, 5 to <15 years and ⩾15 years. Seasonal patterns of age-specific patient usage of healthcare facilities and treatment with intravenous saline for immediate resuscitation in different geographical locations were explored. For this analysis data from two hospitals in Dhaka (DH and MTC) were combined as urban Dhaka. MTC was established to reduce the patient load in DH of icddr,b as well as to ensure early and effective management of severely dehydrating patients in MTC. The city experienced large flooding and a high number of patients in 2007, and after observing an increased patient load during the summer months of 2009, the Ministry of Health and Family Planning, Government of the People's Republic of Bangladesh [Reference Chowdhury29, Reference Khan30] requested icddr,b to establish MTC for treatment of dehydrating diarrhoea cases. However, pathogen-specific diarrhoea and overall socio-demographic characteristics were analysed separately for each facility. For descriptive analysis socio-demographic characteristics (Table 1), and diarrhoea patients and intravenous saline treatment (Fig. 1) were expressed in proportion. χ2 tests were performed to compare differences in results across the sites and seasons in different age groups. Alpha (probability) of <0·05 was considered to be statistically significant.
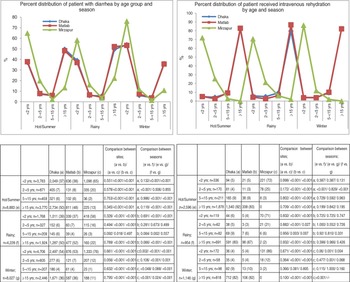
Fig. 1 [colour online]. Percent distribution of patients with diarrhoea and required intravenous rehydration treatment by age group and season in different study areas, 2010–2012.
Table 1. Characteristics of patients in four diarrhoeal treatment facilities of Bangladesh
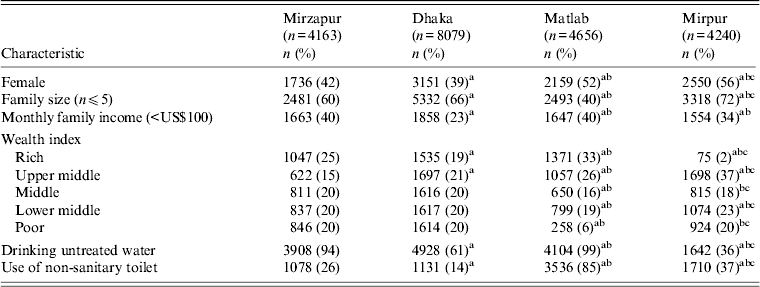
a Comparison between Mirzapur and the other three sites.
b Comparison between Dhaka and Matlab, and Mirpur sites.
c Comparison between Matlab and Mirpur sites.
Data on monthly counts of diarrhoea patients with some/severe dehydration, rotavirus, Shigella, V. cholerae, and ETEC were used to examine the seasonal patterns of each disease in rural and urban settings separately. Mean count of patients with each disease in each site was found to be over-dispersed (variance being much larger than the mean), and quasi-Poisson distribution was appropriate for analysing such a dataset. Hastie & Tibshirani [Reference Hastie and Tibshirani31] developed generalized additive models (GAMs) to blend properties of generalized linear models with additive models for visualizing the relationship. A generalized additive model (GAM) with quasi-Poisson distribution is fitted to examine seasonality, adjusting for secular trend. The model is as follows:
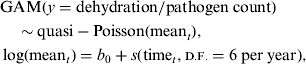 $$\eqalign{& {\rm GAM} (y = {\rm dehydration/pathogen}\;{\rm count}) \cr & \quad \sim {\rm quasi - Poisson(mean}_t ), \cr & \log ({\rm mean}_t ) = b_0 + s({\rm time}_t, {\smallcaps \rm D. F.} = 6\;{\rm per}\;{\rm year}),} $$
$$\eqalign{& {\rm GAM} (y = {\rm dehydration/pathogen}\;{\rm count}) \cr & \quad \sim {\rm quasi - Poisson(mean}_t ), \cr & \log ({\rm mean}_t ) = b_0 + s({\rm time}_t, {\smallcaps \rm D. F.} = 6\;{\rm per}\;{\rm year}),} $$
where t represents time, s is smooth function and d.f. is degrees of freedom. The quasi-Poisson model with smooth function gives the P value for statistical significance of the seasonal pattern of each disease.
Ethical consideration
This study was approved by the Research Review Committee and Ethical Review Committee of icddr,b. All individuals were enrolled and had stool specimens collected for microbiological assessment once they provided informed consent. For infants and children aged ⩽10 years, informed consent was given by their parents or caregivers.
RESULTS
A total of 21 138 diarrhoea patients [KH (n = 4163, 20%), DH (n = 8079, 38%), MTC (n = 4656, 22%), MH (n = 4240, 20%)] irrespective of age and sex, were enrolled in the DDSS during the study period. Forty-four percent of the patients were female. Detailed socio-demographic characteristics are given in Table 1. Rotavirus was the most commonly isolated pathogen [28% (range: KH 27–28%, DH 22–28%, MTC 15–19%, MH 17–21%)], followed by V. cholerae [16% (range: KH 2–4%, DH 10–18%, MTC 4–13%, MH 7–14%)], Shigella [13% (range: KH 11–15%, DH 2–3%, MTC 6–8%, MH 3%)], and ETEC [7% (range: KH 3–4%, DH 7-–1%)]. Children aged <5 years were more frequently infected with rotavirus (80%) followed by Shigella (58%) and ETEC (55%). V. cholerae was often detected in individuals aged ⩾15 years (57%).
The proportion of cases presenting with mixed infections (more than one pathogen) in Mirzapur was 2·8%; while in Dhaka it was 3·5% and in Matlab and Mirpur it was 0·3% and 0·6%, respectively. Of those presenting with V. cholerae in Dhaka, 1% were found to be co-infected with Shigella, 9% with ETEC and 3% with rotavirus. For Mirzapur the proportions were 14%, 5% and 17%, respectively. In Maltab, co-infection with V. cholerae was only found with rotavirus (2%) and in Mirpur it was 2% for both Shigella and rotavirus.
The majority of the patients presented to the facilities during the hot summer months (42%), followed by winter season (38%) of the years during the study period. The rainy season had the lowest recorded rates of diarrhoea-associated hospitalization [KH (n = 718, 17%), DH (n = 1599, 20%), MTC (n = 996, 21%), MH (n = 915, 22%)]. In all three seasons, across all sites, infants aged <2 years were the most frequently represented age group. Despite this, the proportion of infants in total patients peaked during the winter season [KH (76%), urban Dhaka (54%), MH (53%)]. In all study sites, patients aged ⩾15 years composed the next most significant group of patients (Fig. 1). The proportion of patients aged ⩾15 years was lower in KH across all seasons compared to the other three healthcare facilities (Fig. 1). Use of intravenous saline for initial rehydration was greater in patients of older (⩾15 years) age groups for all sites; however, it was highest in Mirzapur in young children (<2 years) for all seasons (Fig. 1).
Dehydrating (some or severe dehydration) diarrhoea was commonly identified in the ⩾15 years age group (48%), followed by young children (38%). Prevalence of individuals presenting with some or severe dehydration was found to exhibit strong seasonal variation which differed across all sites (Fig. 2). In Dhaka, the peak was observed in April and October, in Mirpur it was June and October, while in both Matlab and Mirzapur it was May and October (Fig. 3).
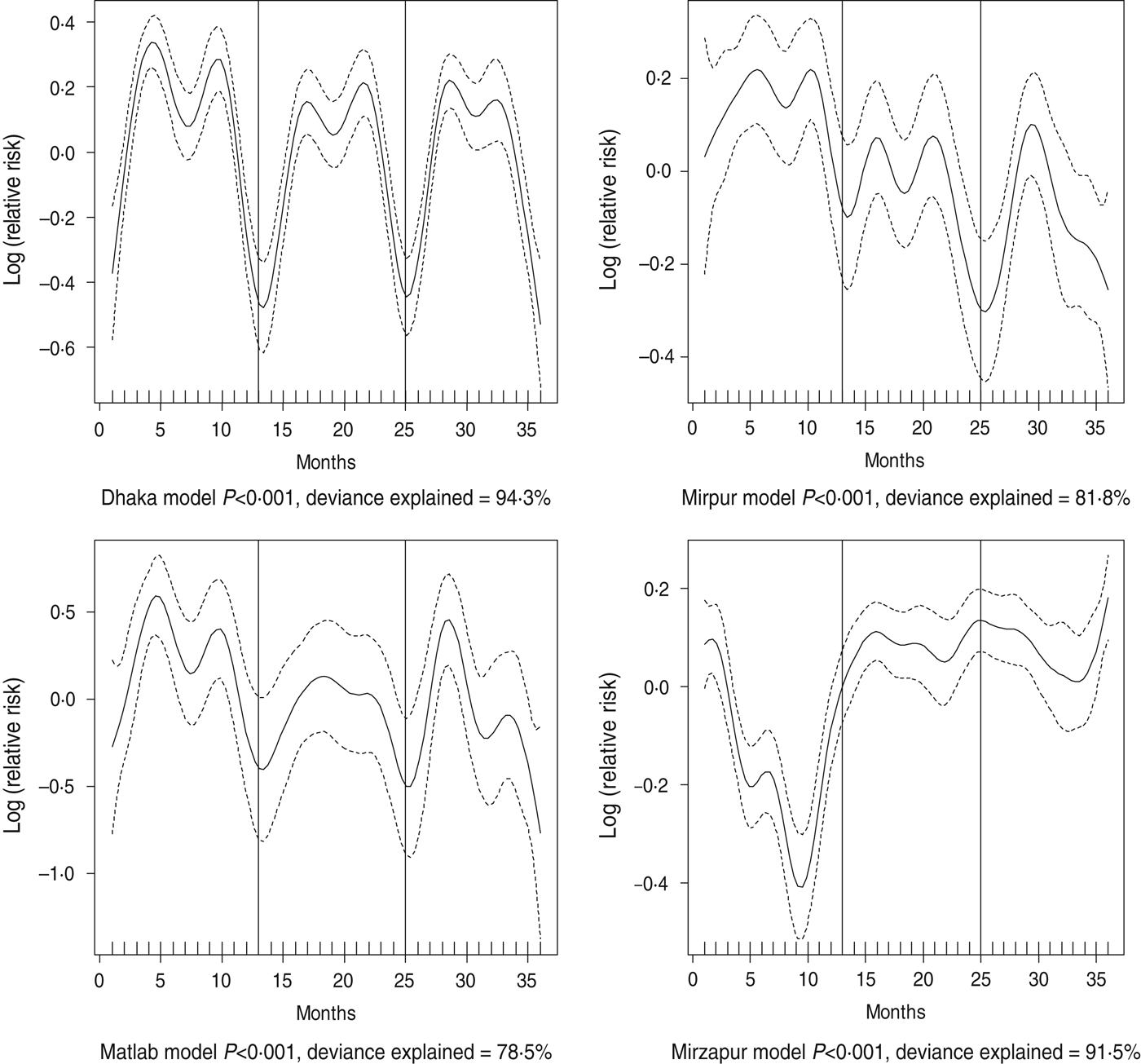
Fig. 2. Seasonality of some or severe dehydration in different study sites in Bangladesh, 2010–2012. Solid line indicates the isolation pattern; dashed lines indicate 95% confidence intervals; vertical lines show the beginning of the year. Deviance explained by predictor as month.
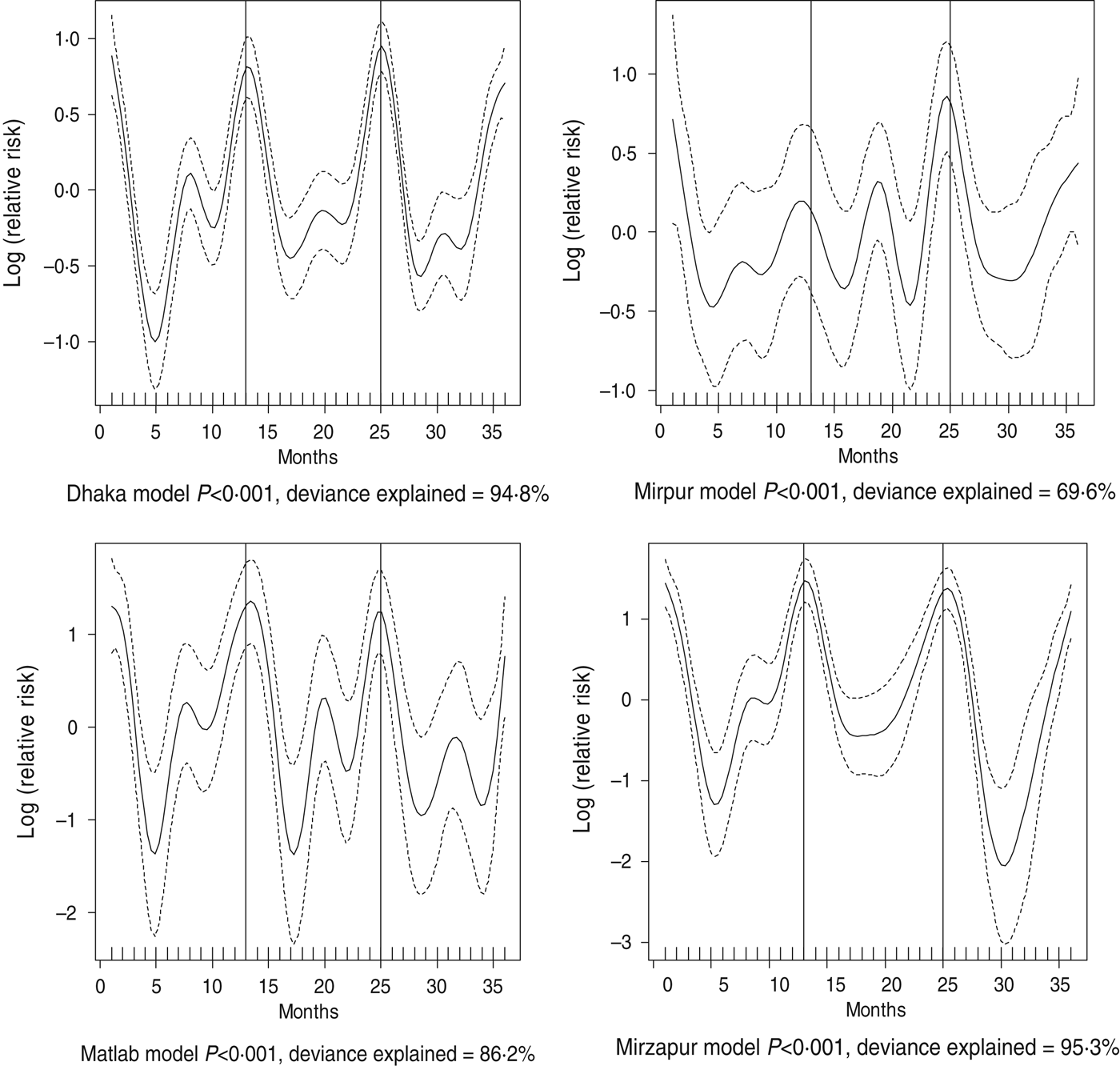
Fig. 3. Seasonality of rotavirus diarrhoea in different study sites in Bangladesh, 2010–2012. Solid line indicates the isolation pattern; dashed lines indicate 95% confidence intervals; vertical lines show the beginning of the year. Deviance explained by predictor as month.
For all four healthcare facilities, the number of rotavirus diarrhoea cases per month showed significant seasonal variation. Seasonality identified a high burden in the winter months across the sites (December, January) with low burdens in May (Dhaka), August (Mirpur) and July (Matlab) (Fig. 3). Shigella infections showed distinct seasonal variation in Dhaka and Mirzapur only, peaking in April–May and October (Fig. 4). Seasonal patterns were also inconsistent for cholera. Cholera peaks were identified in Dhaka and rural Matlab in the hot summer months of March and April and during the rainy season of September and October (Fig. 5). However, no seasonal variation was found in Mirpur and Mirzapur. ETEC diarrhoea was detected in half the sites in Dhaka and Mirzapur, with seasonality evident in Dhaka during the summer months of April–June (Fig. 6).
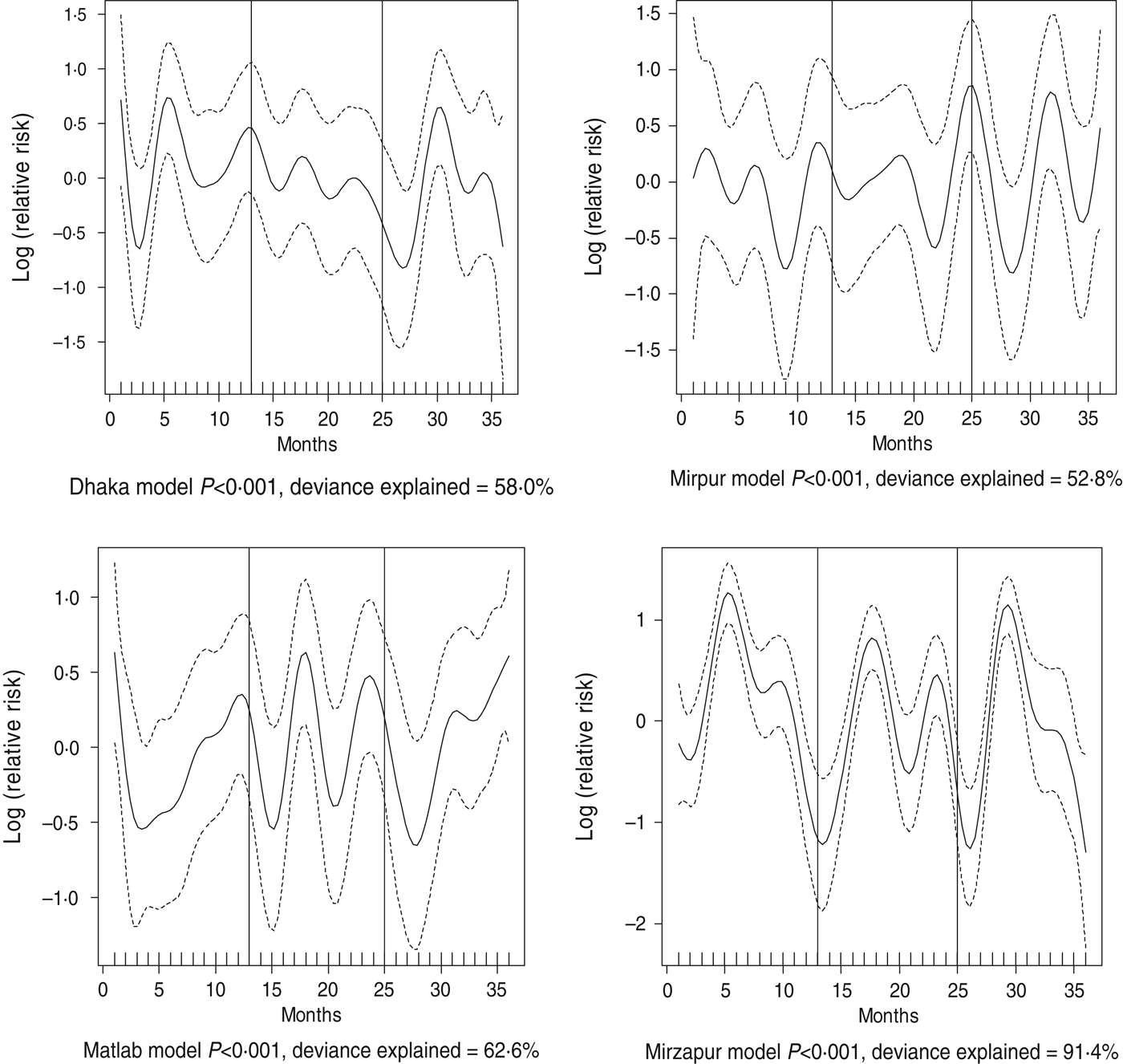
Fig. 4. Seasonality of shigellosis in different study sites in Bangladesh, 2010–2012. Solid line indicates the isolation pattern; dashed lines indicate 95% confidence intervals; vertical lines show the beginning of the year. Deviance explained by predictor as month.
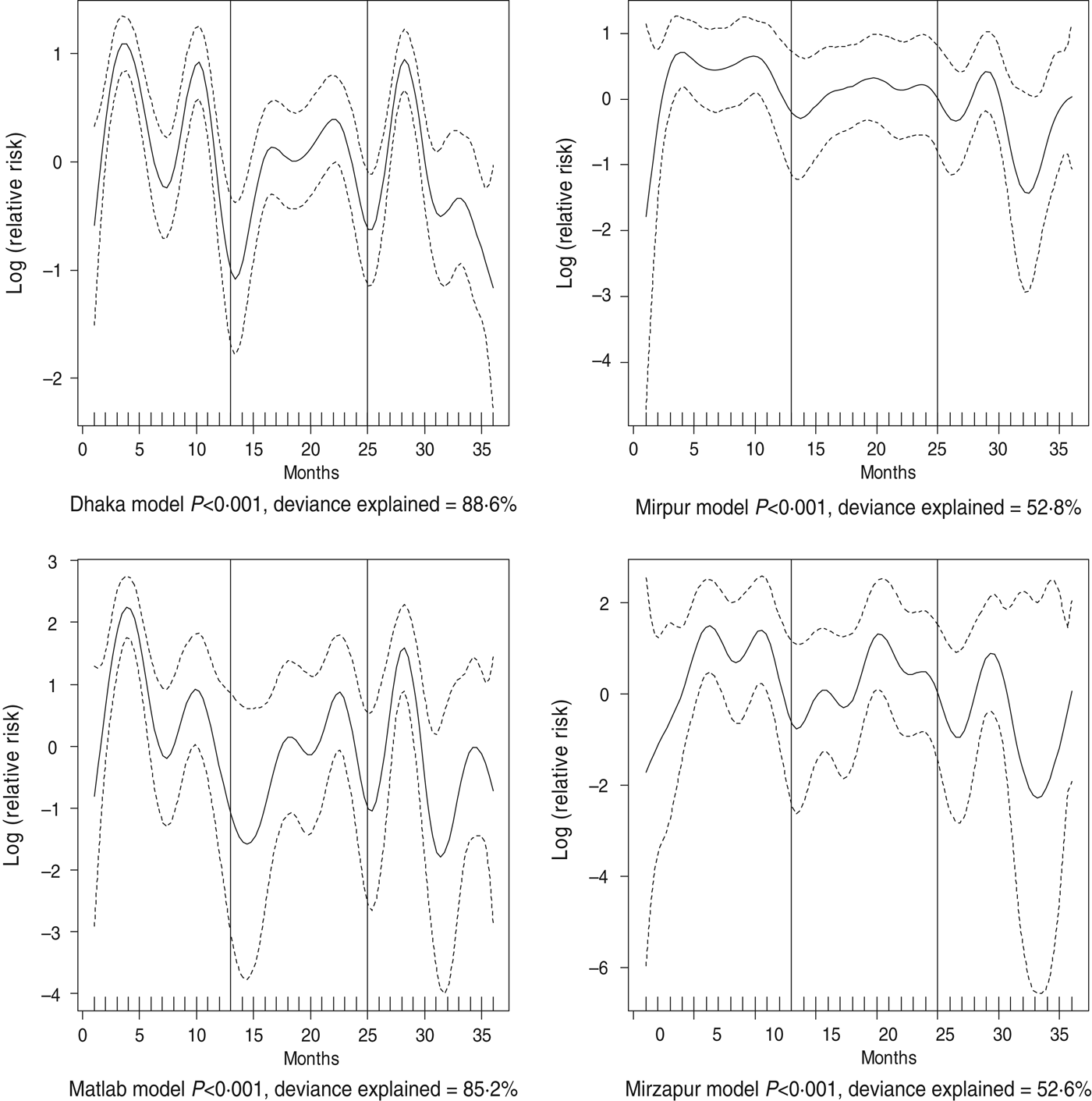
Fig. 5. Seasonality of cholera in different study sites in Bangladesh, 2010–2012. Solid line indicates the isolation pattern; dashed lines indicate 95% confidence intervals; vertical lines show the beginning of the year. Deviance explained by predictor as month
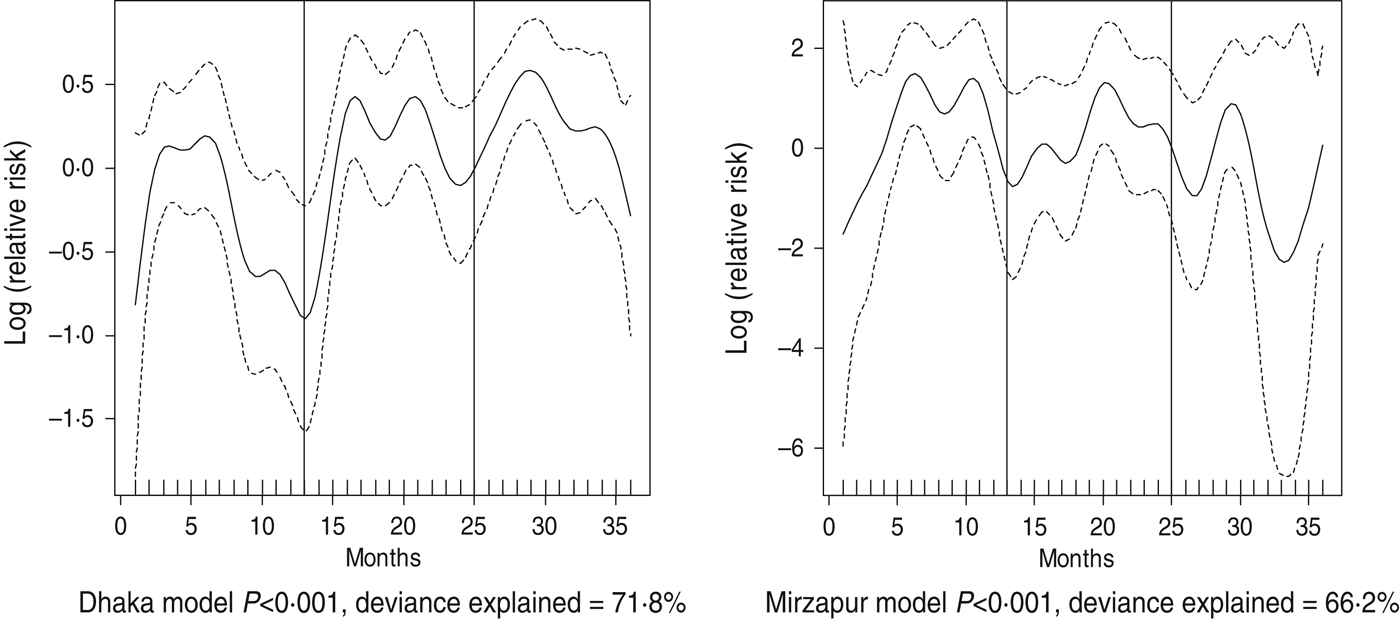
Fig. 6. Seasonality of ETEC diarrhoea in Dhaka and Mirzapur, 2010–2012. Solid line indicates the isolation pattern; dashed lines indicate 95% confidence intervals; vertical lines show the beginning of the year. Deviance explained by predictor as month.
DISCUSSION
The aim of the study was to examine the seasonal differences in the distribution of aetiological agents throughout the year in different geographical areas. The results indicate that overall diarrhoea incidence peaks during the hot and winter seasons. Such seasonal variations in diarrhoea might be due to associations with environmental factors, e.g. rainfall, ambient temperature and occupational behaviour of people in each season as reported previously [Reference Checkley32, Reference Singh33]. On the other hand, improvement in hygienic behaviours and hand washing, also associated with seasonal diversity as well as geographical heterogeneity, showed that increased water availability often has a greater effect in reducing diarrhoea incidence as opposed to improving the water quality alone [Reference Pinfold, Horan and Mara34]. Insufficient access to water is a common phenomenon in urban areas including Dhaka. Although this may be an important explanation for higher incidence of diarrhoeal illness during these two non-rainy seasons, the present study did not aim to determine such an association.
Rotavirus was the most important cause of diarrhoea throughout the year in young children, with an increase in the frequency of isolation during the cooler months. V. cholerae is the second most common cause of diarrhoea followed by Shigella and ETEC. Children aged <2 years formed the largest group reporting diarrhoea across all utilized surveillance systems. Rotavirus and ETEC were the most commonly isolated pathogens in this age group, in line with previous studies [Reference Rowland35, Reference Bittencourt36]. Regarding ETEC, one study observed infections peaked during the warmer months in rural Bangladesh [Reference Khan19]; however, in the present study this strong seasonality was observed in urban Dhaka only. The incidence of ETEC diarrhoea in infants has been shown to be associated with the frequency of consumption of weaning foods contaminated with faecal coliforms. The seasonal peak of ETEC diarrhoea witnessed in this study coincides with the period when food is more likely to be contaminated due to temperatures associated higher bacterial growth [Reference Rowland35]. Rotavirus, on the other hand, showed two peaks, one in the winter months and another in the rainy season across the sites. Rural Mirzapur was the only site where rotavirus seasonality was observed, especially during December.
Patients aged ⩾15 years constituted around a third of the total number of enrolled cases. This age group was more likely to suffer from dehydration and more frequently required intravenous fluids for correction of dehydration. Additionally, they were more commonly infected with V. cholerae and ETEC. The higher rates of dehydration could be explained by the different dominant diarrhoea-causing species – with V. cholerae known to cause severe acute diarrhoea often associated with severe dehydration and hypovolaemic shock. It could, however, also be explained by biases associated with healthcare-seeking behaviours for adults vs. children. Many adults will postpone seeking medical treatment until necessary owing to the cost of forfeiting employment-generating activities, or childcare activities [Reference Ahmed37]. Adults presenting to treatment centres may not therefore be representative of the total diarrhoea-affected adult population. Oral rehydration therapy (ORT) may prevent dehydration and make home management possible for most cases thus reducing the necessity for hospitalization, causing adults to be underrepresented in the actual diarrhoeal disease population.
The results from the study revealed strong geographical variations in Bangladesh with regard to the seasonality of diarrhoea. Cholera, for example, can be seen to have strong environmental determinants, which are particularly evident in Dhaka as well as in Matlab where seasonal variation is strong. Simultaneously, however, no seasonal variation was observed for cholera incidence rate in rural Mirzapur and also in urban Mirpur. Such a pattern remains unaccounted for at this point in time. Mirpur is considered as one of the highest-density population areas in Dhaka, the capital city, with higher number of cholera patients reporting to the hospital [Reference Wahed38]. This could be the after-effect of recent intervention of oral cholera vaccine [39]. The study revealed seasonal geographical heterogeneity of Shigella. Rural Mirzapur and urban Dhaka witnessed high burdens of illness during the hot summer (April and June), and late rainy (September) and winter (November) months. We do not have any explanation for these findings and thus recommend future research to examine these relationships further. Different cultural attitudes towards water, sanitation and hygiene, host characteristics, immunity against putative pathogens, changing virulence properties of the organism itself, smaller environmental fluctuations at the site and changes in disease transmission dynamics could all be underlying factors.
Limitations
Hospital-based data may not adequately represent the sick population at large in the community. However, with tropical monsoon-type climate in general, and a hot and rainy summer as well as a dry winter without any notable geographical diversity, it might be assumed that the study sites and large sample might be representative of other areas of Bangladesh in terms of seasonality of the pathogens. Our study's strengths were that we followed an unbiased systematic sampling irrespective of age, sex, nutritional status, disease severity or socioeconomic context; observed isolation patterns in different geographical locations; and used a large dataset for this analysis. The present study considered only four major pathogens responsible for diarrhoea to determine the seasonal variations. There might be other enteropathogens that follow distinct seasonality. Lack of meteorological variables such as temperature, rainfall, water level, etc. which potentially correlate with diarrhoea were not tested. Furthermore, this paper did not investigate factors that affect seasonality; rather, this study examined whether the month of the year impacts the rate of laboratory-confirmed cases of ETEC, V. cholerae, Shigella spp., and rotavirus in Bangladesh. However, despite variations over the study period at different sites, some differences were not statistically significant, possibly due to lack of power with small sample size.
CONCLUSION
Understanding of the seasonal variations of enteric pathogens allows clinicians to determine appropriate therapy, and policy-makers to decide about selection of antimicrobial therapy and targeting of resources. Periods of high disease burden can be extremely disruptive to health systems – drawing staff and resources away from other areas to meet demand. To facilitate continuation of the expected standards of care, facilities may need to recruit additional staff and procure additional supplies. The data provided by this study is paramount in facilitating an understanding of environmentally driven diarrhoeal illnesses in Bangladesh. This knowledge, in combination with modelling and meteorological data, could be utilized to predict the likelihood of an upsurge in cases of diarrhoea of a particular aetiology in a given place at a given time. This could help to decrease diarrhoea incidence, reduce transmission as well as fatality. For Bangladesh and other developing countries where diarrhoeal diseases still represent a major health problem, efficient allocation of resources is the best option to deal with probable frequent outbreaks. Such knowledge is important as it can help policy-makers in formulating appropriate and targeted preventive and control strategies prior to seasonal upsurges of diarrhoea. At the same time, we recommend follow-up research for the implementation of the findings.
ACKNOWLEDGEMENTS
The research protocol was funded by the Swedish International Development Cooperation Agency (Sida), grant no. MD-90020 and GR-900599. icddr,b acknowledges with gratitude the commitment of Sida to its research efforts. Hospital surveillance was funded by icddr,b and the Government of the People's Republic of Bangladesh to icddr,b research efforts. icddr,b acknowledges with gratitude the commitment of the Government of the People's Republic of Bangladesh to the Centre's research efforts. icddr,b also gratefully acknowledges the following donors who provide unrestricted support to the Centre's research efforts: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID).
DECLARATION OF INTEREST
None.










