Short report
Raw drinking milk (RDM) has a diverse microbial flora which can include pathogens transmissible to humans. In the UK, RDM is most commonly sourced from cows, and to a lesser extent from goats, sheep and buffalo (https://acmsf.food.gov.uk/sites/default/files/acm_1269_revised_final.pdf). Pathogens most commonly associated with human illness following the consumption of RDM are Campylobacter spp., Salmonella spp., Brucella melitensis, Mycobacterium bovis, tick-borne encephalitis virus and Shiga Toxin-producing Escherichia coli (STEC) [1]. Contamination can arise from direct excretion into the milk from animals with systemic infection as well as from localised infections, such as mastitis, and faecal contamination during milking or from the wider farm environment [1].
In England and Wales, RDM can currently only be sold by registered RDM producers directly to the customer at the farm gate or farmhouse catering operation, by farmers at farmers' markets, distributors using a vehicle as a shop such as a milk round, direct online sales or vending machines at farms. In England and Wales, RDM must be labelled with a health warning. In England, this includes the statement ‘This milk has not been heat treated and may contain organisms harmful to health’, in Wales this is expanded to include ‘The Food Standards Agency strongly advises that it should not be consumed by children, pregnant women, older people or those who are unwell or have a chronic illness’. In Scotland, the sale of RDM is banned.
Systematic, national surveillance of general outbreaks of intestinal infectious disease (IID) in England and Wales has been undertaken by Public Health England (PHE) since 1992 [Reference Wall2]. Upon notification of an outbreak, a standardised surveillance form is sent to the consultant in communicable disease control leading the investigation with a request that it is completed once the outbreak investigation has ended. Since 2004, following an EU direction in 2003 (Directive 2003/99/EC), reporting of outbreaks of IID to PHE's electronic foodborne and non-foodborne gastrointestinal outbreak surveillance system (eFOSS) has been mandated.
Reported data on foodborne IID outbreaks are valuable for analysing links between foodborne illness and specific food vehicles or situations that cause them, monitoring trends and assessing risk. While the number of cases linked to confirmed and putative vehicles during outbreak investigations do not portray the true burden of disease, they can be useful in examining trends which may be indicative of risk exposures amongst apparently sporadic cases [Reference Adams3]. These data can, therefore, contribute to risk assessments and inform policy. Information from eFOSS is routinely provided to UK government departments, including the Food Standards Agency, the Department for Environmental, Food and Rural Affairs and the Department of Health; and to European agencies, specifically the European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC).
Previous reviews of foodborne outbreaks in England and Wales between 1992 and 2008 described an overall decline in the number of foodborne outbreaks detected [Reference Gormley4]. Despite this overall decline in foodborne outbreaks, studies published in 2003 and 2005 [Reference Gillespie5, Reference Gillespie6] highlighted the emergence of STEC O157:H7 as a milk borne pathogen, and the role of RDM in causing outbreaks of human disease in England and Wales. A subsequent review of foodborne outbreaks in 2011 [Reference Adams3] described a decrease in the number of outbreaks caused by the consumption of milk and milk products, including those made from raw milk, during the study period. In that study, most milk borne outbreaks were caused by STEC O157:H7 followed by Salmonella and Campylobacter species [Reference Gormley4]. However, a decline in the relative role of RDM as a causative agent of outbreaks of STEC O157:H7 was reported between 2002 and 2012 [Reference Adams3].
The aim of this short report is to provide an update on the previously published data on outbreaks and incidents involving reported human illness associated with RDM [Reference Gormley4, Reference Gillespie5, Reference Gillespie6]. In this report, we use the term RDM to include RDM and dairy products containing RDM, such as cheese and cream. Here, we review all the outbreaks and incidents involving reported human illness associated with RDM, between 1992 and 2017 in England and Wales. In addition, we describe available surveillance data on sporadic STEC infections and sporadic cases of listeriosis in relation to exposure to RDM.
Between 1992 and 2001, there were 19 outbreaks (1.7 per year) linked to RDM involving 229 people (20.8 cases per year); 36 of these were hospitalised as a result of the infection (Table 1). At least one outbreak occurred every year, except in the years 1999 and 2001. The highest number of outbreaks recorded in one year was three, as observed in the years 1993, 1994, 1996 and 2000. The number of cases declined from 72 in 1992 to nine in 2002 (Table 1). No outbreaks associated with RDM were identified between 2003 and 2013.
Table 1. Reported foodborne IID outbreaks and IID outbreaks associated with RDM or products made using raw milk, in England and Wales, 1992–2017
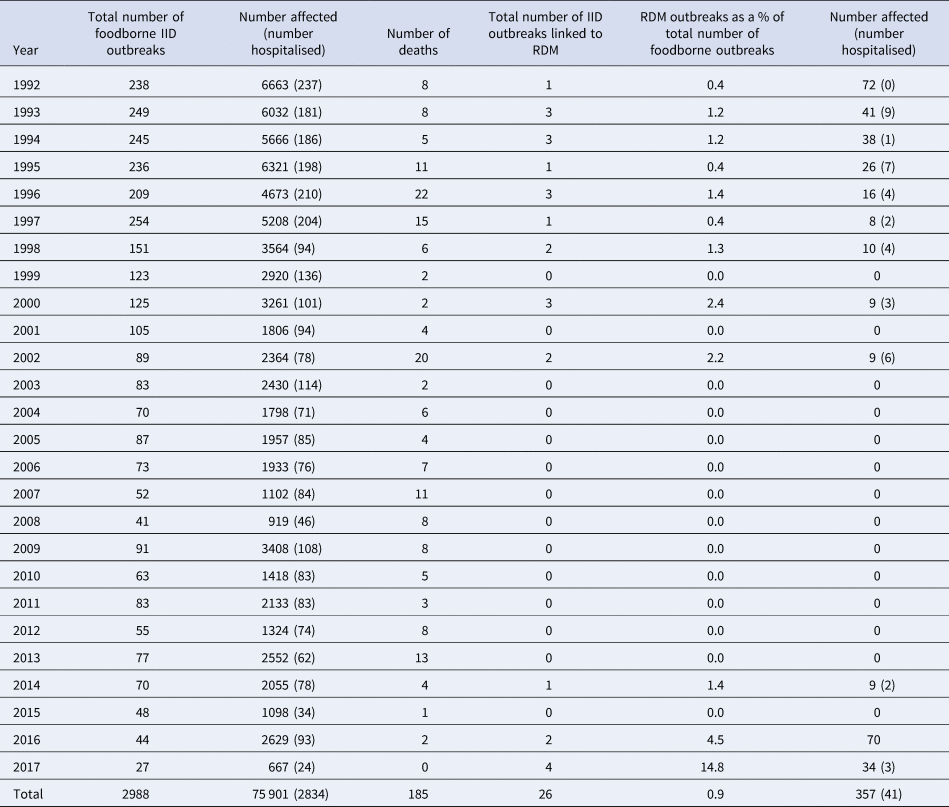
More recently between 2014 and 2017, seven outbreaks (1.75 per year) caused by RDM have been investigated and reported, involving 114 people (28.5 cases per year), five reported hospitalisations and one death (Table 2). The seven outbreaks were caused by E. coli O157:H7 (n = 3) or Campylobacter jejuni (n = 4). In 2017, four outbreaks were recorded, the highest since systematic data collection on outbreaks began in 1992 (Tables 1 and 2). In addition, two incidents of IID (Listeria monocytogenes, n = 1; Salmonella Dublin, n = 1) were investigated which each involved one case, with epidemiological and microbiological links to RDM, but were not designated as outbreaks under the definition (Directive 2003/99/EC). Where data were available, additional details on the number and ages of children affected are provided in Table 2. In total 18 (32.1%) of 54 laboratory confirmed cases were in children aged under 16 years. RDM is not recommended for consumption by children.
Table 2. Outbreaks/incidents associated with RDM in England and Wales, 01/01/2014 to 20/12/2017a

a A Food-borne outbreak is defined ‘an incidence, observed under given circumstances, of two or more human cases of the same disease and/or infection, or a situation in which the observed number of human cases exceeds the expected number and where the cases are linked, or are probably linked, to the same food source’ (Directive 2003/99/EC).
b U, unknown.
c Identical on WGS, refers to isolates that fall within 0 and five single nucleotide polymorphisms of each other.
d Probable cause of death.
During the time frame of this study, there were 12 outbreaks associated with pasteurised milk, including 10 caused by pasteurisation failures (Campylobacter species n = 3; Salmonella species n = 3; STEC O157:H7 n = 3; Cryptosporidium species n = 1), and two (both Campylobacter species) caused by post-pasteurisation contamination of the milk. The last milk borne outbreak linked to pasteurisation failure occurred in 2011 [Reference Fernandes7].
In England, national enhanced surveillance systems exist for STEC and Listeria and collect detailed, standardised exposure information using an enhanced surveillance questionnaire (ESQ) on every case, which can be used to observe risk factors over time [Reference Byrne8, https://www.gov.uk/government/publications/listeria-enhanced-surveillance-questionnaire]. Both ESQs include a question relating to the consumption of RDM, in the exposure period. Analysis of the ESQ's for cases reported between 1st May 2015 and 20th December 2017, identified 19/1284 (1.48%) sporadic cases of STEC (cases related to outbreaks were excluded) and 13/535 (2.43%) sporadic cases of listeriosis reported exposure to RDM. It is not possible to use ESQ data to provide a measure of risk associated with the consumption of RDM, or products made using raw milk, for sporadic cases, because cases are often exposed to more than one potential risk factor for infection, and it is not possible to confirm that consumption of RDM caused the symptoms. However, this analysis does give some indication of the potential of RDM as a vehicle of transmission. Self-reporting may lead to an underestimate; parents may withhold information on their family's consumption of RDM as it is contraindicated for consumption by children. For other pathogens, including Campylobacter and non-typhoidal Salmonella, collection of exposure data is subject to local variation, the information is not gathered into a central enhanced surveillance database, and it is not possible to assess RDM exposures amongst cases.
Since 2015, the use of routine whole genome sequencing (WGS) at PHE has provided unprecedented sensitivity and accuracy in identifying microbiologically linked cases of infection [Reference Butcher9, Reference Treacy10]. However, all RDM-associated STEC O157:H7 outbreaks reported to date have been detected through epidemiological links established by local health protection teams prior to the availability of the WGS results [Reference Butcher9, Reference Treacy10]. At the time of the STEC O157:H7 RDM outbreak in 2014, human and RDM isolates were linked using multilocus variable number tandaem repeat analysis, although WGS was used retrospectively to definitively link cases to the outbreak strain [Reference Butcher9]. WGS was also used for case ascertainment in the 2016 and 2017 outbreaks, and for definitively linking RDM to clinical cases in the 2017 outbreak [Reference Treacy10].
In the UK, reducing foodborne IID has been a key target in the Food Standard's Agency's (FSA) strategy on foodborne disease since its inception in 2000. In July 2015, controls governing the sale and marketing of RDM were reviewed by the FSA and at that time no changes were recommended to the existing control measures (http://www.food.gov.uk/sites/default/files/multimedia/pdfs/board/boardpapers2014/fsa-140704.pdf). Existing control measures include microbiological sampling and testing, appropriate labelling and restrictions on the sale and marketing of RDM. The restrictions on the sale of RDM are governed by the Food Hygiene (England) Regulations (2006) (http://www.legislation.gov.uk/uksi/2006/14/pdfs/uksi_20060014_en.pdf) which includes a microbiological standard of plate count at 30 °C ⩽20 000 cfu/ml and coliforms at <100 cfu/ml. However, a recent assessment of the microbiological quality and safety of RDM on the retail sale in England between 2014 and 2016, found that pathogens and/or indicators of poor hygiene were present in almost half of samples examined [Reference Willis11]. These results demonstrate the importance of continued monitoring and maintaining strict controls on the production and sale of this product.
The data presented within this review indicate that the risk of IID from RDM has likely increased over the last decade, with an increase in the number of outbreaks and incidents associated with RDM since 2014, following an eleven-year period where no RDM outbreaks were reported. Despite the labelling requirements and recommendations that children should not consume RDM, almost a third of outbreak cases were children. Alongside this, there has been an increase in consumer popularity and in registered RDM producers in the UK. In January 2018, there were 165 sites registered for the production of RDM for public consumption, compared to April 2014 when there were 107 registered RDM producers (ref – https://acmsf.food.gov.uk/sites/default/files/acm_1269_revised_final.pdf).
At the Advisory Committee on the Microbiological Safety of Food (ACMSF) (https://acmsf.food.gov.uk/committee/acmsf/acmsfmeets/acmsfmeets/acmsf-meeting-10-may-2018) meeting in May 2018, it was recognised that the microbiological risk associated with consumption of RDM in the UK had increased, reflecting greater levels of exposure due to the increased number of registered producers and volume of consumption, alongside and an increase in the number of outbreaks in human illness associated with RDM. The FSA has subsequently set out additional recommendations to enhance existing controls around registration and hygiene of RDM producers and as of February 2019, is undertaking a timely consultation on the controls (https://www.food.gov.uk/business-guidance/raw-cows-drinking-milk) and provide advice on RDM to consumers (https://www.food.gov.uk/safety-hygiene/raw-drinking-milk). The FSA advises that raw or unpasteurised milk and cream may contain harmful bacteria that cause food poisoning. Pregnant women, infants and small children, elderly people and people with a compromised immune system such as cancer patients, are particularly vulnerable to food poisoning and should not consume raw milk. Severe symptoms of gastrointestinal disease are seen more frequently in younger children, and there is a higher risk of children developing HUS following infection with STEC [Reference Byrne8].
In England and Wales, outbreaks linked to the consumption of RDM are rare. The recent increased occurrence, albeit in low frequency, of RDM-related IID incidents is noteworthy, and of concern, and emphasises the continued role of RDM as a cause for human illness. The decision to consume RDM should be informed by the scientific, evidenced-based assessment of the associated risks to public health.
Acknowledgements
We would like to thank David Alexander and all our colleagues in the FSA policy team for reviewing and commenting on the manuscript. The opinions and conclusions expressed in this article are solely the views of the author(s) and do not necessarily reflect those of the Food Standards Agency.
Financial support
This work was supported by the National Institute for Health Research Health Protection Research Unit in Gastrointestinal Infections. Natalie Adams and Claire Jenkins are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with PHE, in Oxford and the Institute of Food Research. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.




