INTRODUCTION
Hepatitis B virus (HBV) is a hepatotropic non-cytopathic DNA virus belonging to the family Hepadnaviridae; HBV infection is a major public health burden worldwide [Reference Koumbi1, Reference Wang, Chen and Zhu2]. Globally, there are more than 240 million chronic carriers of HBV who are at high risk of developing severe sequelae, such as end-stage cirrhosis and hepatocellular carcinoma [Reference Wang, Chen and Zhu2]. In the most highly epidemic areas, especially mainland China, mother–fetus transmission of HBV plays an important role in the high prevalence of carrier status [Reference Wang, Chen and Zhu2–Reference Op den Brouw4]. This results in a high rate (90%) of chronic infection when the infant acquires HBV through mother–infant transmission. The increased incidence of chronicity is attributed to the immaturity of the neonatal immune system and, especially, to the functional impairment of T cells [Reference Koumbi1].
Dendritic cells (DCs) are the most important professional antigen-presenting cells (APCs) which play an important role in antiviral immunity and have the unique capacity to activate naive T cells, and stimulate B and natural killer cells [Reference Op den Brouw4–Reference Banchereau and Steinman6]. The importance of DCs has been demonstrated by experiments showing that neonatal T cells could achieve adult-like responses when stimulated by isolated allogeneic adult DCs [Reference Guy7], while the alteration of quantity and function of DCs in neonates can lead to responsive defects of adaptive T cells to foreign antigens. The main dysfunctions of neonatal DCs include low circulating numbers, low levels of co-stimulatory molecule expression, decreased induction of cytokine production, and decreased capacity to stimulate naive T cells [Reference De8, Reference Velilla, Rugeles and Chougnet9].
Toll-like receptor 3 (TLR3) is a type of pattern recognition receptor (PRR) which is closely associated with virus cleaning and links innate and adaptive immunity [Reference Wu, Zhang and Huang10, Reference Palm and Medzhitov11]. Previous studies suggested that TLR3 was preferentially expressed by myeloid dendritic cells (mDCs) and recognized virus-derived double-stranded RNA (dsRNA), which would activate the antigen-presenting function of mDCs after combining with dsRNA [Reference Velilla, Rugeles and Chougnet9, Reference Aoshi12]. Therefore variation of TLR3 expression levels would inevitably impact the function of mDCs.
Koumbi et al. found that there was no difference in the quantity of peripheral blood DCs between uninfected neonates of hepatitis B surface antigen (HBsAg)-positive mothers and healthy neonates of healthy mothers, and plasmacytoid dendritic cell (pDC) function secreting alpha interferon (IFN-α) as well [Reference Koumbi1]. However, it was reported that in the liver and peripheral blood of HBV-infected individuals, HBV DNA levels were 1 × 109 to 1 × 1010 copies/ml and HBsAg titres were 100 μg/ml, resulting in interaction between virus and DCs, e.g. low levels of co-stimulatory molecule expression of mDCs, decreased capacity to stimulate naive T cells [Reference Op den Brouw4] and hepatitis B e antigen (HBeAg), which because of the small size might transverse the placenta and induce T-cell tolerance [Reference Wang, Chen and Zhu2, Reference Milich and Liang13]. Currently, it is not clear whether HBeAg and HBV DNA, which may enter the uterus during pregnancy, cause injury to neonatal DC frequency and expression levels of peripheral blood mononuclear cell (PBMC) TLR3. The aim of the present study was to investigate whether the frequencies of DCs and the expression of PBMC TLR3 may be altered in neonates of HBsAg-positive mothers with different HBV serological profiles.
MATERIALS AND METHODS
Study population
All of the 144 neonates recruited in the study were from the Third People's Hospital of Taiyuan, during July 2011 to April 2013. All the neonates were born to HBsAg-positive mothers who were HCV- and HIV-negative, with normal liver function test (LFT). The mothers had not received any antiviral treatment during pregnancy.
All neonates were full-term babies (between 37 and 42 gestation weeks) with a birth weight >2500 g. According to national recommendations, all neonates received one dose of 200 IU hepatitis B immune globulin (HBIG) and their first recombinant hepatitis B vaccine (NCPC GeneTech Biotechnology Co. Ltd, China) within 24 h after birth. Recombinant hepatitis B vaccination was completed with a further two doses of vaccine at ages 1 and 6 months.
Femoral venous blood sample of neonates was obtained before the administration of passive-active immunoprophylaxis. Peripheral blood samples from the mothers were obtained before delivery and all blood samples were collected using heparinized syringes. Non-anticoagulant peripheral blood samples from neonates (before the administration of passive-active immunoprophylaxis) and mothers (before delivery) were also collected.
The research protocol was approved by the ethics committees of Shanxi Medical University (2010032), and all mothers provided written informed consent.
Serological HBV markers and HBV DNA
HBsAg and HBeAg were measured using chemiluminescence immunoassay (CLIA) kits (Roche Co. Ltd, Switzerland) for all mothers during pregnancy, and their neonates on the day of delivery before the administration of passive-active immunoprophylaxis. HBV DNA levels of mothers and neonates were determined by fluorescence quantitative polymerase chain reaction (PCR) assay (Da'an Gene Co. Ltd, Sun Yat-Sen University, Guangdong, China). HBV DNA loads >1 × 103 copies/ml were defined as positive.
HBV intrauterine transmission was defined as finding HBsAg and/or HBV DNA positive in the peripheral blood of neonates within 24 h after birth, before active or passive immunoprophylaxis [Reference Li14].
Circulation DC frequencies
The frequencies of mDCs and pDCs were determined by using four-colour flow-cytometric analysis. Peripheral blood was collected in EDTA tubes and was processed within 6 h of collection. Whole blood was incubated with a mixed-lineage cocktail (Lin) of fluorescein isothiocyanate (FITC)-conjugated antibodies, which included antibodies to CD3, CD14, CD16, CD19, CD20, and CD56; peridinin chlorophyll protein (PerCP)-conjugated antibody to HLA-DR; phycoerythrin (PE)-conjugated antibody to either CD11c or CD123; isotype-matched antibodies were used as controls. All monoclonal antibodies were from eBioscience (USA). After 15 min at room temperature in the dark, erythrocytes were lysed with 2 ml red blood cell lysis buffer for 10 min at room temperature in the dark. After washing with phosphate-buffered saline (PBS) twice, the cells were fixed in 1% paraformaldehyde. In each case, the cells from 1 × 105 events were acquired on a FACSort Flow Cytometer (Becton Dickinson, USA). The cells were gated by forward and side scatter characteristics, and data analysis was performed using Cellquest software (Becton Dickinson). Circulating mDCs were defined as Lin negative, HLA-DR positive, and CD11c positive (Fig. 1), and pDCs were defined as Lin negative, HLA-DR positive, and CD123 positive (Fig. 1). The absolute numbers of mDCs and pDCs were calculated using the percentage of cells with respect to number of PBMCs.

Fig. 1 [colour online]. Representative phenotypic analysis of myeloid dendritic cell (mDC) and plasmacytoid dendritic cell (pDC) subsets by flow cytometry. (a) Peripheral blood mononuclear cells were gated according to their forward and side scatter characteristics (region R1). (b) Lin-negative but HLA-DR-positive cells were gated on region R2. (c, d) Gates R1 and R2 were selected, and mDCs were identified as Lin negative, HLA-DR-positive and CD11c-positive (c), while pDCs were identified as Lin negative, HLA-DR-positive and CD123-positive (R3) (d).
PBMC TLR3 expression quantity
Peripheral blood was processed within 6 h from the time of blood collection and delivery. PBMCs were isolated by centrifugation on Ficoll-Histopaque (Hao Yang Biological Manufacturing Co. Ltd, China). The PBMCs were divided into a test group and an isotype control group after washing twice with PBS. The PBMCs of the test group were incubated with mouse monoclonal antibody (Santa Cruz Biotechnology Inc., USA) for 30 min at 4°C. Next, cells of two groups were incubated with FITC-conjugated goat anti-mouse IgG for 30 min at room temperature in the dark. After washing twice with PBS, the cells were fixed in 1% paraformaldehyde. In each case, the cells from 1 × 105 events were acquired on a FACSort Flow Cytometer (Becton Dickinson). The expression levels of TLR3 were the differences in value between the test and isotype control groups (Fig. 2).

Fig. 2 [colour online]. Representative expression levels of peripheral blood mononuclear cell Toll-like receptor 3 (PBMC TLR3) by flow cytometry. (a) PBMCs were gated according to their forward and side scatter characteristics. (b, c) TLR3 values of the isotype control and test groups, respectively.
Statistical analysis
Differences in numeric variables between the two groups were analysed by non-parametric Mann–Whitney test with two-tailed P values. Correlation analysis was performed by χ 2 test. The results were expressed as the mean ± standard error of the mean (s.e.m.), and a P value of <0·05 was considered statistically significant. Statistical calculations were performed using SPSS v. 19 (SPSS Inc., USA).
RESULTS
HBV markers of study population
The incidence of HBV intrauterine transmission was 9·72% (14/144). In the HBV intrauterine transmission group (n = 14), ten HBeAg-positive and two HBV DNA-positive neonates were found. There were 45 HBeAg-positive neonates in the HBV intrauterine non-transmission group (n = 130) of which none was HBV DNA positive. Of the 144 HBsAg-positive mothers, the proportion HBeAg positive, HBV DNA positive and HBeAg/HBV DNA double-positive were 44·4% (64/144), 50·7% (73/144) and 40·3% (58/144), respectively. Of the HBV DNA-positive mothers, 40 had a high HBV DNA load (HBV DNA >1 × 107 copies/ml) and 33 a low HBV DNA load (1 × 103 copies/ml <HBV DNA ⩽1 × 107 copies/ml).
HBV intrauterine transmission and neonatal DC and TLR3 frequencies
Neonatal mDC frequencies of the HBV intrauterine transmission group were higher compared to the HBV intrauterine non-transmission group, and the frequencies of pDCs between these two groups were reversed. But there were no statistically significant differences of neonatal mDCs (Z = − 1·18, P = 0·238) and pDCs (Z = − 0·408, P = 0·683) (Table 1, Fig. 1). The expression levels of PBMC TLR3 of neonates in the intrauterine transmission group were higher compared to the HBV intrauterine non-transmission group, but no statistically significant difference was found (Z = − 0·083, P = 0·934) (Table 1, Fig. 2).
Table 1. Relationship between HBV intrauterine transmission and neonatal DC frequencies and TLR3 expression levels

Values given are number ± standard error of the mean.
HBV, Hepatitis B virus; DCs, dendritic cells; mDCs, myeloid dendritic cells; pDCs, plasmacytoid dendritic cells, TLR3, Toll-like receptor 3.
* Z = − 1·18, P = 0·238.
† Z = − 0·408, P = 0·683.
‡ Z = − 0·083, P = 0·934.
Relationship between maternal HBeAg positivity and neonatal HBeAg positivity
Of the 144 neonates in our study, 55 were HBeAg positive and 54 of the mothers were also HBeAg positive; neonatal HBeAg positivity was closely related to maternal HBeAg positivity [odds ratio (OR) 6·32, P < 0·001, 95% confidence interval (CI) 3·575–11·174]. The contingency coefficient was 0·648 (P < 0·001).
Considering that both neonatal HBeAg positivity and maternal HBeAg positivity were related to maternal HBV DNA positivity, we conducted a stratification analysis among mothers who were HBV DNA positive or negative. We found that the relationship between maternal HBeAg positivity and neonatal HBeAg positivity was statistically significant when maternal HBV DNA was positive (maternal HBV DNA negative: OR 1·969, 95% CI 0·884–4·386, P = 0·001; maternal HBV DNA positive: OR 8·286, 95% CI 4·137–16·596, P < 0·001) (Table 2).
Table 2. Stratified analysis of association between maternal HBeAg positivity and neonatal HBeAg positivity

HBV, Hepatitis B virus.
* Continuity correction χ 2 test.
† Fisher's exact test.
Maternal HBeAg and neonatal DC and TLR3 frequencies
The DC frequencies of neonates born to HBeAg-positive mothers were similar to those of neonates born to HBeAg-negative mothers (mDCs: Z = − 0·263, P = 0·792; pDCs: Z = − 0·123, P = 0·902) (Table 3). The difference of PBMC TLR3 expression levels between neonates born to HBeAg-positive and HBeAg-negative mothers was not statistically significant (Z = − 0·394, P = 0·693) (Table 3).
Table 3. Frequencies of neonatal DCs and expression levels of neonatal TLR3 in HBsAg-positive mothers with different HBV serological profiles
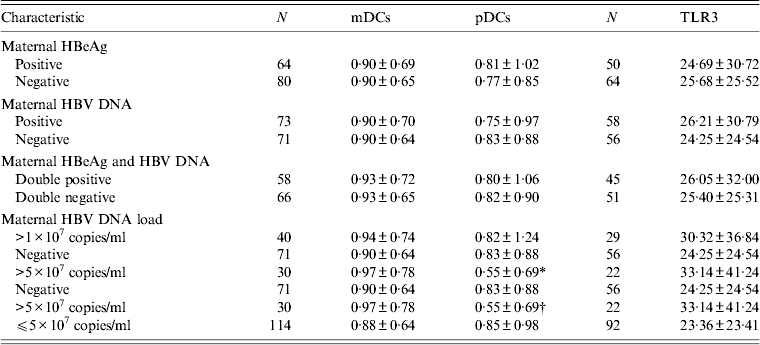
Values given are number ± standard error of the mean.
HBV, Hepatitis B virus; DCs, dendritic cells; mDCs, myeloid dendritic cells; pDCs, plasmacytoid dendritic cells; TLR3, Toll-like receptor 3.
* Z = − 1·981, P = 0·048.
† Z = − 2·170, P = 0·03.
Maternal HBV DNA and neonatal DC and TLR3 frequencies
The pDC frequencies of neonates born to HBV DNA-positive mothers were lower than those of neonates born to HBV DNA-negative mothers, but no statistically significant difference was found (Z = − 1·019, P = 0·208). TLR3 expression levels of neonates of HBV DNA-positive mothers were higher compared to neonates of HBV DNA-negative mothers, but no statistically significant difference was found (Z = − 0·119, P = 0·905). There were no significant differences in frequencies of neonatal DCs and expression levels of neonatal TLR3 between HBeAg/HBV DNA double-positive and double-negative mothers (mDCs: Z = − 0·163, P = 0·871; pDCs: Z = − 0·433, P = 0·665; TLR3: Z = − 0·18, P = 0·857) (Table 3).
Comparing neonates whose maternal HBV DNA loads were >1 × 107 copies/ml with neonates whose maternal HBV DNA was negative, the quantitative differences of neonatal DCs were not significant (mDCs: Z = − 0·043, P = 0·966; pDCs: Z = − 1·167, P = 0·243). Although the difference of neonatal TLR3 levels was not significant, the difference value (D value) of neonatal TLR3 expression levels between these two groups was greater than that between neonates whose maternal HBV DNA was positive and negative (Table 3).
Compared to neonates born to HBV DNA-negative mothers, the frequencies of neonatal pDCs decreased significantly (P < 0·05) when maternal HBV DNA loads were >5 × 107 copies/ml, and they were also significantly lower than those of neonates whose maternal HBV DNA loads were ⩽5 × 107 copies/ml (P < 0·05). The differences in neonatal mDC frequencies and TLR3 expression levels were not significant (mDCs: Z = − 0·052, P = 0·959; TLR3: Z = − 0·806, P = 0·42). However, the D value of TLR3 expression levels between neonates whose maternal HBV DNA loads were >5 × 107 copies/ml and negative was greater than that between neonates whose maternal HBV DNA loads were >1 × 107 copies/ml and negative (Table 3).
DISCUSSION
Neonates born to HBsAg-positive mothers, as a special population, are in danger of immunity failure by immunoprophylaxis and chronic infection by HBV; this group has therefore attracted more attention from physicians and public health experts regarding whether they have HBV intrauterine transmission or not. These neonates have much more opportunities for contact with maternal HBV antigen and particles at the fetus stage than other neonates born to healthy mothers, therefore maternal HBV antigen and particles may impair the neonatal immune system and cause dysfunction of the immune system throughout life [Reference Wang, Chen and Zhu2].
Immaturity of innate immunity could explain neonatal vulnerability to infection [Reference Koumbi1]. In the majority of studies, the frequencies and function of neonatal DCs has been shown to be defective compared to adult DCs [Reference Duan3, Reference De8, Reference Zaghouani, Hoeman and Adkins15]. DCs had been proven to play an important role in the immune response of body, and alteration of the frequencies and function of DCs will affect immune response to pathogens [Reference Banchereau and Steinman6, Reference Cervantes-Barragan16]. TLR3, the PRR which is closely associated with infection and cleaning of virus, and which links innate and adaptive immunity [Reference Wu, Zhang and Huang10, Reference Palm and Medzhitov11, Reference Lanzavecchia and Sallusto17], is preferentially expressed on mDCs. There are two major distinct subsets in human peripheral blood, mDCs that activate naive T cells and secrete high levels of interleukin-12 (IL-12) [Reference Duan3], and pDCs, known as interferon-producing cells (IPCs), that can produce up to 1000 times more IFN-α than any other cell type when challenged with certain inactivated viruses [Reference Duan3]. We speculated that DC and TLR3 frequencies in neonates born to HBsAg-positive mothers with different HBV serological profiles would be different.
Comparing neonates with HBV intrauterine transmission to those with HBV intrauterine non-transmission, there were no significant differences in DC and TLR3 expression levels. However, the quantities of mDC and TLR3 levels were both clearly increased. Several previous studies have found the quantity and function of pDCs in chronic hepatitis B patients to be damaged [Reference Zhang18], and mDCs as well as TLR3 to be closely related to antigen uptake and presentation [Reference Medzhitov19]. Therefore mDC and TLR3 levels in neonates with HBV intrauterine transmission might be altered because the viral antigen loads in neonates with HBV intrauterine transmission were higher than the loads in non-transmission neonates. However, in our study only two neonates with HBV intrauterine transmission were HBsAg/HBV DNA double-positive, the others were HBsAg single-positive, which might have resulted from blood contamination during delivery rather than viral replication inside newborns. Koumbi et al. also demonstrated that the frequencies of DC subsets in total PBMCs in neonates born to HBsAg-positive mothers were similar to those observed in neonates of healthy mothers [Reference Koumbi1]. Further evidence is required to confirm this.
HBeAg positivity is a sign of active HBV replication. In our study maternal HBeAg positivity was shown to be a risk factor for neonatal HBeAg positivity, which was illustrated by neonates whose maternal HBeAg was positive having more opportunities to be HBeAg positive, and maternal HBV DNA positivity may enhance the relationship between maternal HBeAg and neonatal HBeAg positivity. This consequence was in accord with previous studies showing that maternal HBeAg could enter the uterus by transversing the placental barrier and that maternal HBeAg positivity was a risk factor for HBV intrauterine transmission in neonates [Reference Xu20–Reference Dwivedi22]. Therefore we speculated that maternal HBeAg might influence neonatal frequencies of DCs and PBMC TLR3 expression levels.
There were no significantly quantitative changes of DCs and TLR3 in neonates born to HBeAg-positive mothers compared to those born to HBeAg-negative mothers. No quantitative differences of neonatal DCs and TLR3 between HBeAg/HBV DNA double-positive and negative mothers were found. Although several researchers have suggested that maternal HBeAg could transverse the placental barrier to infect the fetus and further activate neonatal mDCs and impact their function of antigen-presenting [Reference Wang, Chen and Zhu2, Reference Untergasser5, Reference Milich and Liang13, Reference David23, Reference Arima24], in our study all the subjects came from a hospital for infectious diseases, when HBeAg loads were too high intervention measures according to national recommendations could have been introduced, therefore the maternal HBeAg loads might not be enough to affect the neonates.
In the present study, the quantitative differences of neonatal DCs were not statistically significant between HBV DNA-positive and -negative mothers, but the level of neonatal pDCs was lower in HBV DNA-positive mothers. When maternal HBV DNA loads were >5 × 107 copies/ml, the frequency of neonatal pDCs was significantly lower than for neonates whose maternal HBV DNA loads were ⩽5 × 107 copies/ml and negative. As is known, pDCs are innate immune cells that sense virus-derived nucleic acids through TLR7 and TLR9 and respond with massive IFN-I production [Reference Cervantes-Barragan16, Reference Gilliet, Cao and Liu25, Reference Martinet26]. HBV DNA positivity was the decisive indicator for active viral replication, and the replication levels of virus rapidly increase when maternal HBV DNA loads are >5 × 107 copies/ml, producing much more viral particles which might transverse the placental barrier [Reference You27]. Therefore the neonatal pDCs, which are immature, may be severely exhausted when they have early contact with viral particles [Reference Vincent28].
Compared to neonates whose maternal HBV DNA was negative, the expression levels of TLR3 increased clearly in neonates whose maternal HBV DNA was positive, >1 × 107 copies/ml or >5 × 107 copies/ml, respectively, but did not reach statistical significance. Moreover, the D value of TLR3 expression levels between maternal HBV DNA loads >5 × 107 copies/ml and negative was more than that between loads >1 × 107 copies/ml and negative. It is suggested that compared to the negative group, neonatal TLR3 expression gradually increased in company with maternal HBV DNA loads increasing. PBMC TLR3 is able to recognize virus-derived dsRNA and initiate a signal cascade reaction which activates the antigen-presenting function of DCs after combining with dsRNA [Reference Aoshi12, Reference Zhang29, Reference Montero and De Andres Martin30], Therefore the quantity of dsRNA, which is the intermediate product of the replication of HBV DNA, is increased when HBV replicates severely and neonatal TLR3 expression is then activated. We hypothesize that the more severe the replication, the higher the neonatal TLR3 expression will be, but more evidences is required to confirm our hypothesis.
In conclusion, the present study proves that maternal HBeAg positivity is a risk factor for neonatal HBeAg positivity. It suggests that the maternal HBeAg can transverse the placenta from mother to fetus. Maternal HBV DNA loads >5 × 107 copies/ml might be an influencing factor for variation of neonatal pDC frequencies. However, it was only the frequencies that were a focus of our study. It would useful to include the assessment of the function and in vitro test of neonatal DCs in neonates born to HBsAg-positive mothers with different HBV serological profiles, but this requires high serum and blood volumes which are impossible to obtain for neonates. Our purpose is simply to provide a theoretical foundation and clues for further studies from the epidemiological viewpoint, such as mechanism research regarding HBV intrauterine transmission and failure of hepatitis B vaccine; these findings should be verified by in vitro experiments and larger studies.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (grant no. 81072341) and Returned Personnel Science Research Project of Shanxi Province, China (grant no. 2008-50). We are particularly grateful to the study subjects and their mothers. We also acknowledge the nursing staff of Obstetrics and Gynaecology Department, the Third People's Hospital of Taiyuan City, Shanxi, P.R. China for their assistance and the Department of Physiology, Key Laboratory of Cellular Physiology, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi P.R. China for their technical support. We gratefully acknowledge Dr Lijian Lei and Dr Junni Wei for a critical review of this manuscript.
DECLARATION OF INTEREST
None.








