Introduction
Wildlife populations may be important sources of infectious diseases for humans and other animal species. The susceptibility of wild animals to develop disease upon infection varies between species. Many wildlife hosts are known to carry silent infections, not showing clinical signs of disease (reservoirs). The presence of seropositive animals may however indicate the presence of the infectious agent in the ecosystem. Tularaemia, caused by the bacterium Francisella tularensis, is an important zoonotic disease in Sweden. According to the Swedish Public Health Agency, human infection is related to outdoor activity and hunters have a higher than average prevalence. The number of human cases varies widely between years during the last decade, the highest number recorded was 759 in 2015, and the lowest 84 in 2017 as presented in the database of the Public Health Agency [1]. The host range of F. tularensis is very broad with more than 300 species susceptible to infection [Reference Mörner, Addison, Williams and Barker2]. There are several known routes of infection in humans: vectors (mosquitos, ticks), ingestion, inhalation and direct or indirect contact. All these routes are possible also for wildlife, but infection through ingestion might be of greater importance in predators and scavengers. The wildlife species most commonly associated with tularaemia in Sweden are the mountain hare (Lepus timidus) and the European brown hare (Lepus europaeus), species that in previous studies have been shown to develop acute disseminated disease, quickly leading to death [Reference Mörner3, Reference Hestvik4]. In central Europe, the disease in the European brown hare is described as either acute or subacute-chronic [Reference Gyuranecz5, Reference Decors6]. Other wildlife species prone to develop acute disease are a variety of small rodent species, conversely, other species such as wild boar (Sus scrofa) and raccoon dogs (Nyctereutes procyonoides) are considered relatively resistant [Reference Olsufjev and Pavlovsky7, Reference Sjöstedt and Tärnvik8]. Even relatively resistant animals that hunt or scavenge on carcasses of infected animals risk getting infected and may mount an antibody response. Several studies suggest that raccoon dogs, red foxes (Vulpes vulpes) and wild boar may be useful sentinels of tularaemia [Reference Hoflechner-Poltl9–Reference Otto12]. Antibodies have also been detected in Japanese black bears (Ursus thibetanus japonicus) and Japanese raccoon dogs (Nyctereutes procyonoides viverrinus) [Reference Sharma13]. In one Austrian study, the bacterium F. tularensis was found in the submandibular lymph nodes of red foxes [Reference Hofer14].
This study is an investigation of seven predator or scavenger species: brown bear (Ursus arctos), Eurasian lynx (Lynx lynx), raccoon dog, red fox, wild boar, wolf (Canis lupus) and wolverine (Gulo gulo). These animal species were selected as they may be infected but are considered less susceptible to develop disease. The aim was to investigate the seroprevalence, and to assess if a rapid, simple and affordable serological test could be useful for screening wild predators and scavengers. An additional aim was to investigate if F. tularensis is present in selected lymph nodes of seropositive animals, indicating latent infection. These investigations could give information on whether these species may serve as sentinels for the presence, enhancement and/or spread of tularaemia in Sweden.
Methods
Material
Six-hundred-and-fifty-six animals were sampled in 2011–2015. They had either been sent in for post-mortem examination at the Swedish National Veterinary Institute or were sampled at hunting. All brown bears and wolverines had been shot during hunting or killed in traffic accidents, the raccoon dogs were killed in a national eradication programme [15]. They were all necropsied and showed no signs of disease. Of the Eurasian lynx, red fox, wild boar and wolf, 3/34, 12/119, 2/248 and 4/59, respectively had died from disease which was recorded when necropsied. None of the diseased animals had antibodies against F. tularensis. The diseases were not related to tularaemia, the most common diseases being sarcoptes (Eurasian lynx, red fox and wolf) and pyothorax (red fox). The remaining animals had not shown any signs of disease, they had been hunted, killed in traffic accidents or originated from research studies and studies evaluating restraining traps [16]. Samples taken were blood, and when possible, lymphatic tissue (tonsils and submandibular lymph nodes, Table 1).
Table 1. Number of animals of each species of predators and scavengers from which samples of blood, tonsils and submandibular lymph nodes were investigated
The investigated brown bears, wolves and Eurasian lynx originated from the central and northern parts of Sweden, the wolverines and raccoon dogs from the northern parts. The wild boar originated from the central parts of the country, and the red foxes from central and southern parts. Tularaemia occurs in almost the whole country but has a patchy distribution and is more common in the northern and central parts. Statistics on human case reports in the database of the Public Health Agency show occasional cases as far south as the counties of Kronoberg, Blekinge and Skåne, but they are very sporadic and few in these areas [1]. Out of the animals included in this study, 27 of the 119 red foxes were from the southern county Kronoberg. The remaining animals originated from northern and central parts where tularaemia is more common.
Serology
To detect F. tularensis antibodies, slide agglutination and microagglutination were performed on all blood samples. In the slide agglutination test, an F. tularensis antigen was used (Bioveta a.s., Ivanovice na Hané, Czech Republic). The test can be used with whole blood. According to the manufacturer's instructions, a 40 µl sample was mixed with 200 µl (dilution ratio 1:6) of the F. tularensis solution and the result was read after 1–3 min. A positive result was defined as the presence of agglutination flakes and clarification of the surrounding solution. For evaluation of the test, eight dog sera with the known titres 0, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640, previously established with the tube agglutination test (routine diagnostics, SVA), were tested with slide agglutination. Positive results were obtained in the slide agglutination test for titres 1:80 and higher, the dog sera with titres 1:40 and lower were negative. Based on these results, the limit of detection for the slide agglutination test was determined as a titre of 1:80. The method was therefore modified, and an equal volume of sample and antigen solution, 1:2 (sample:antigen solution), was used. This resulted in detection of titres as low as 1:10 when testing the dog sera.
For the microagglutination, an in-house developed protocol from Wageningen Bioveterinary Research (WBVR), Wageningen, the Netherlands was used. F. tularensis non-viable bacteria in 0.5% formaldehyde with addition of crystal-violet (Becton Dickinson AB, Stockholm, Sweden) was used as antigen. The antigen was diluted in saline solution in the ratio of 1:15 to create a working solution. Eighty microliters of antigen working solution was mixed with 20 µl of sera in the first row of a 96-well V-bottom microtitre plate (VWR International, Stockholm, Sweden). Fifty microlitre of the mixture was transferred to the next row, containing 50 µl of the working solution. This procedure was repeated to the last row, causing a two-fold dilution in each step and the titres to be tested 1:5, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640. A known positive serum (Germaine Laboratories, San Antonio, United States) and a negative rabbit serum (Bio Jet Service, Uppsala, Sweden) were used respectively as positive and negative controls for each plate. The plate was sealed (VWR International, Stockholm, Sweden) and incubated for 21 ± 1 h at 37 °C. The wells were examined visually using a light box. Because of the subjective nature of the reading, two persons read and agreed on each sample. A serum dilution was considered negative if a blue spot was visible in the well. To evaluate the reading of the microagglutination at SVA a ring test was performed. Five of the sera of various antibody titres from the Swedish wild animals were also tested at Wageningen Bioveterinary Research (WBVR), Wageningen, the Netherlands. Five dog sera from WBVR with variable antibody titres were also sent to SVA for testing.
Since Brucella sp. antibodies cross-react with F. tularensis antigen [Reference Behan and Klein17], samples with positive F. tularensis antibody titres were tested for Brucella antibodies using the Rose Bengal Test (Microbiology department, SVA). Since Brucella sp. does not occur in Sweden, cross reactivity with Brucella sp. was only tested on the smaller subset of samples where antibodies against Francisella had been detected at microagglutination.
Real-time PCR, histopathology and immunohistochemistry
For animals in which F. tularensis antibodies were present in the slide agglutination test, and for which submandibular lymph nodes and tonsils were available, real-time polymerase chain reaction (PCR) analysis was performed on pooled samples of submandibular lymph node and tonsil from each animal. For animals in which F. tularensis was detected, the PCR was also performed on samples from the spleen. Sample preparation, DNA extraction and real-time PCR were performed as described previously [Reference Hestvik18]. Pieces of submandibular lymph nodes and tonsils were fixed in 10% neutral buffered formalin, embedded in paraffin and routinely processed for histopathology and immunohistochemistry (IHC). For animals positive with the PCR, tissue sections were stained with haematoxylin and eosin (HE). To detect and visualise F. tularensis IHC was applied using a mouse primary monoclonal antibody, FB11 (Meridian Life Science Inc., Nordic Biosite AB, Täby, Sweden) directed against F. tularensis (modified from [Reference Hestvik18] in that in the present study the primary antibody was diluted at 1:200).
Results
Serology
The slide agglutination test was performed on all 656 samples using two ratios for serum:antigen solution; 1:6 and 1:2. The reason for also including a modified ratio was that the antibody titres in predator and scavengers are expected to include individuals with low titres. Using the ratio 1:6, recommended by the manufacturer, one brown bear and one wild boar were positive. Using the ratio 1:2, 34 animals were positive as shown in Table 2, divided by species. The geographic locations of the positive animals are shown in Figure 1.
Table 2. Number of positive animals in the slide agglutination test. Ratio serum:antigen solution was 1:1

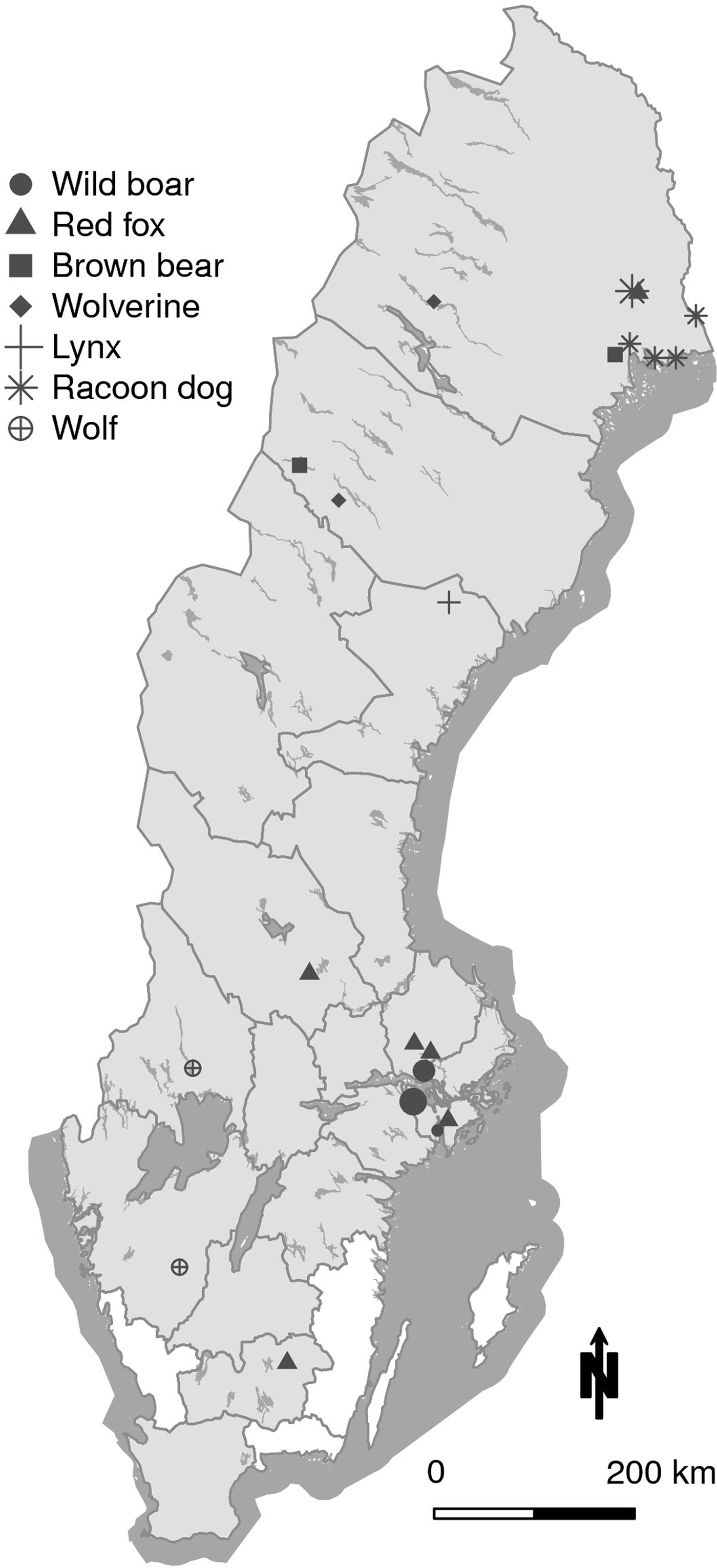
Fig. 1. Map of Sweden showing the geographical location of the 34 animals seropositive in the F. tularensis slide agglutination test. In wild boar, larger circles indicate four and nine animals respectively, found in the same location. In the racoon dog, the larger star indicates three animals in the same location. All other markings represent individual animals. The counties from where the 656 sampled animals originated are shaded in grey.
The microagglutination test was performed on all 656 samples (Table 3). Since many of the samples had variable degree of haemolysis, the lowest readable titre varied between samples. The remnants of the red blood cells obscured the bottom of the wells and made it impossible to read the result at low titres. A cut-off level for a positive serum in these wildlife species that are assumed not to develop disease and to have low titres could presumably be set to 1:10 [Reference Sharma13]. Since this titre level was not possible to read for the majority of samples, the results will be useful just for establishing that most of these animals had low titres, if any. It is plausible that in this study animals with low titres remained undetected due to the low quality of their serum samples. At the highest detected titre, 1:80, all 656 sera were possible to evaluate. At titre 1:40 and 1:20, 651 and 559 sera respectively were possible to read. Since only 357 samples were readable at titre 1:10 the limit for evaluating a sample was set to titre 1:20. The result of the microagglutination test is shown in Table 4; one red fox, one brown bear, one wolverine and nine wild boar showed positive results in one or more serum dilutions, indicating the presence of F. tularensis antibodies at a titre of 1:20 or higher. A second microagglutination test confirmed the results of the 12 positive samples, all of them also testing positive in the slide agglutination test.
Table 3. F. tularensis microagglutination, showing the lowest readable titre for each wildlife species

Table 4. Number of positive animals per antibody titre in the F. tularensis microagglutination test

Five seropositive samples (one brown bear, two red foxes and two wild boar) with variable serological levels and variable degree of haemolysis were also sent to WBVR for confirmation of the microagglutination test. Results from SVA and WBVR were similar, with readings identical or differing in one titre step, which is considered satisfactory. Only one serum sample (from a brown bear) differed with two titre steps, with the higher titre 1:320 reported by WBVR. For the dog sera sent from WBVR to SVA, the readings were identical or differing in one titre step.
To exclude cross-reaction with Brucella antibodies, serology for Brucella sp., the Rose Bengal Test, was performed on 10 of the 12 sera positive at microagglutination where the serum volume was sufficient. Seven samples from wild boar and one red fox were negative, the other three (brown bear, wolverine and wild boar) were not suitable for examination due to haemolysis.
Real-time PCR for detection of F. tularensis in lymph nodes
From the 34 animals with positive titres, tonsils and submandibular lymph nodes were available for 12 (two brown bears, six red fox, two wolves and two wolverines). Additionally, submandibular lymph node, but no tonsil, was available from one raccoon dog. The real-time PCR on pooled samples for each animal detected F. tularensis subsp. holarctica in one brown bear, one red fox and one wolf. To elucidate if bacteria had spread further in the body, samples from spleen were investigated in these three animals but no bacteria could be detected.
Histopathology and immunohistochemistry in lymph nodes
Histopathology and IHC for F. tularensis spp. were applied on the lymph nodes and tonsils for the brown bear, red fox and wolf positive with the real-time PCR. Submandibular lymph nodes were possible to interpret in all three, but the tonsils from the brown bear and red fox were too autolytic to allow interpretation. Histopathology (HE) showed a reactive hyperplasia in the red fox and wolf submandibular lymph nodes, as well as in the wolf tonsil. No reactivity was seen in the brown bear submandibular lymph node. In all three submandibular lymph nodes and in the wolf tonsil, F. tularensis was detected with IHC. The bacteria were primarily located in the cytoplasm of macrophages in both the lymph node cortex and medulla (Fig. 2).
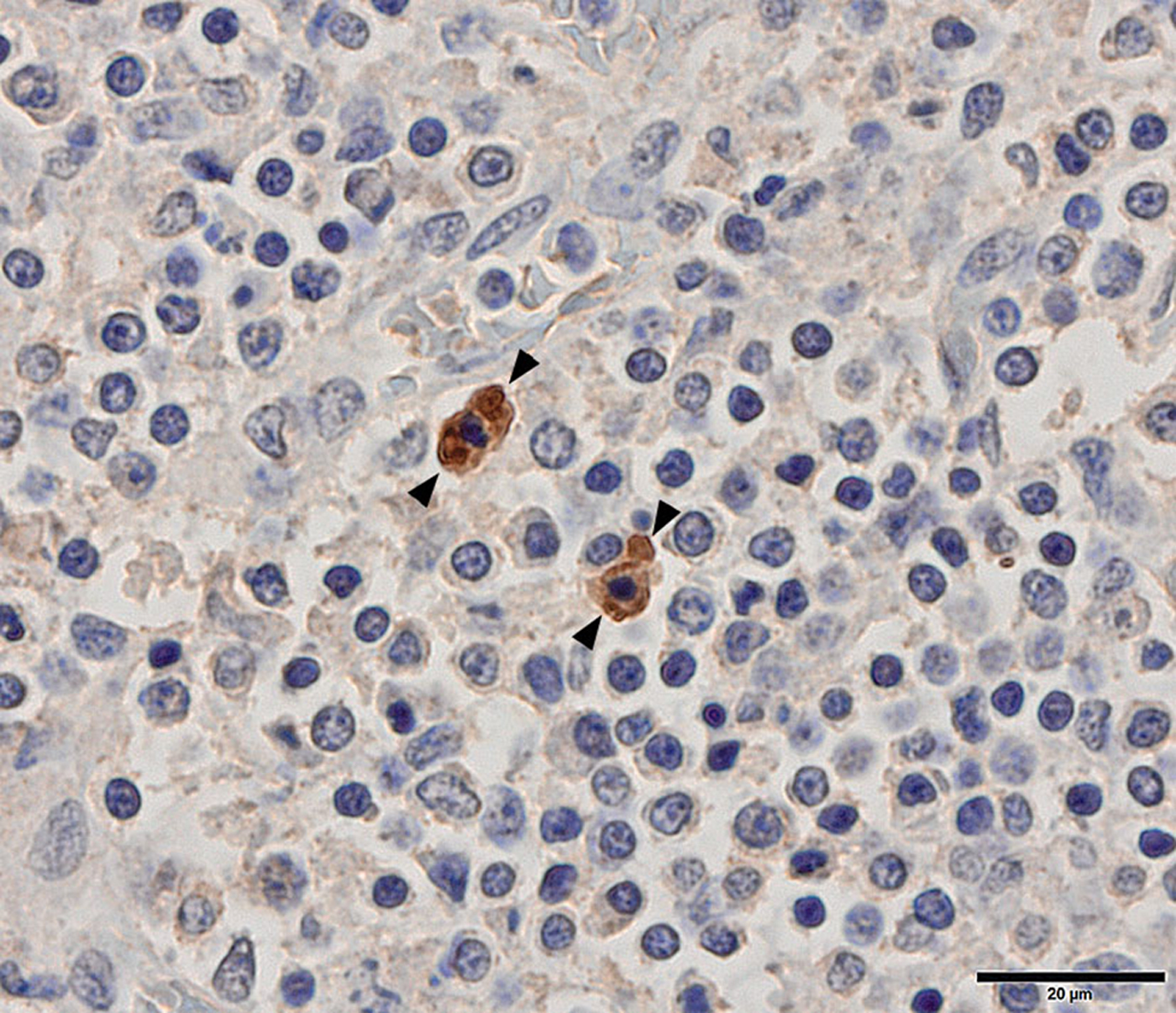
Fig. 2. Tonsil, wolf. Immunohistochemical staining shows the location of F. tularensis bacteria in the cytoplasm of macrophages (between arrowheads), the most frequent location.
Discussion
The main aim of our study was to investigate the suitability of a slide agglutination test for screening wild predators and scavengers for antibodies against F. tularensis, thereby investigating if the selected wildlife species could serve as sentinels for tularaemia. Since such animals are regarded as relatively resistant to infection and disease, the presence of antibodies in serum from wildlife may merely indicate prior contact with the bacterium and not whether animals have suffered from infection. Although positive titres are not necessarily confirming an infection, especially in case of low titres, sero-reactivity in wildlife indicates contact with the bacterium. Tularaemia is present in a large part of the country, has a patchy distribution, and is endemic in many areas in northern and central Sweden. During the last two decades, cases in both humans and hares have been diagnosed increasingly farther south [1, Reference Hestvik4, Reference Eliasson19]. In this study, wild predator and scavenger species were investigated regarding the presence of antibodies against F. tularensis. During a successive spread of tularaemia without obvious outbreaks of disease it is difficult to detect a limited increase of mortality in sensitive animal species in new areas. Since large predators and scavengers may consume large numbers of animals (for example hares and small rodents) it could be efficient to test these species when investigating the presence of this pathogen in a selected area. Many of these animal species are also hunted annually, enabling recurrent sampling and testing of the predator and scavenger populations. The wild boar is present in the middle and southern parts of Sweden, covering both the southern part of the ‘endemic tularaemia area’ and the southern regions where only sporadic human cases have been diagnosed. The red fox and the European lynx inhabit the whole country and could act as indicators to detect the level of tularaemia in endemic areas and spread to new areas. Brown bear and wolverine could be suitable as sentinels for an increase of tularaemia since they inhabit endemic areas in central and northern regions of the county, as data presented at The Swedish Species Information Centre [20]. Wild animals may contract infection with F. tularensis subsp. holarctica via the same routes as humans, i.e. via vectors (mosquitos, ticks), ingestion (via food or water), inhalation and through direct or indirect contact [Reference Anda, Pearson, Tärnvik and Tärnvik21]. However, the risk to get infected through ingestion of infected prey and carcasses is presumably more important in predators and scavengers compared to other taxonomic groups.
Of the investigated species in this study, the Eurasian lynx, brown bear, red fox, raccoon dog, wolf and wolverine are, to a variable degree, predators that may hunt for hares and small rodents. The Eurasian lynx and wolf are carnivores and they commonly prey on roe deer (Capreolus capreolus) and moose (Alces alces), and where available, reindeer (Rangifer tarandus). They may also hunt for various other species, e.g. hares and small rodents [Reference Odden, Linnell and Andersen22]. The wolverine is a predator and scavenger that mainly preys on reindeer but also on small game such as mountain hares, lemmings (Lemmus lemmus) and other rodent species [Reference Persson23]. The red fox is an opportunistic omnivore and a skilled hunter preying on most species up to the size of roe deer fawns, including hares. Carrion is frequently consumed during the winter [Reference Englund24, Reference Dell'Arte25]. Brown bears are omnivores, and ungulates, mainly moose, are important food sources. In addition to hunting, they consume carrion of various animals [Reference Stenset26]. Raccoon dogs are extremely opportunistic omnivores and eat a variety of food including small mammals and vegetables. Carrion is consumed at all times of the year if available, i.e. dead hares or voles [Reference Sutor, Kauhala and Ansorge27]. The wild boar is a generalistic omnivore, the diet to a limited extent includes food of animal origin [Reference Massei, Genov and Staines28, Reference Herrera29].
Methods suitable for surveillance of pathogens such as F. tularensis should be relatively simple and affordable. Based mainly on studies in mouse models, antibody response to F. tularensis mounts approximately two weeks after infection, peaks after one to two months and then declines [Reference Cowley and Elkins30]. Therefore, a suitable screening test must be able to detect low titres. The microagglutination test has been shown to have a high sensitivity and specificity when used on human sera [Reference Sjöstedt and Tärnvik8, Reference Chaignat31]. Since the antibody response of the selected species in the present study is poorly known and the time elapsed between infection and sampling may be long, antibody levels were assumed to be low in animals exposed to the bacterium. This is in accordance with a study on Japanese black bears and Japanese raccoon dogs where antibody titres varied between 1:10 and 1:80 using microagglutination [Reference Sharma13]. In addition, a screening test should ideally be possible to apply to whole blood and haemolytic serum, allowing testing of samples collected during hunting, when haemolysis of blood samples may be common.
The slide agglutination test has successfully been used on a large scale on hares in Hungary for screening during hunting [Reference Gyuranecz32], and in a serological investigation of Austrian hunting dogs [Reference Posautz33]. In our study, the slide agglutination test failed to detect seropositive individuals below titre 1:80 when using the ratio of blood:antigen solution according to the manufacturer. When adjusting the ratio to 1:2, the number of positive animals increased since titres as low as 1:10 could be detected. Using this ratio, the slide agglutination test is suitable as a screening method for the predators and omnivores investigated.
The microagglutination test only detected 12 of the animals seropositive in the slide agglutination test, this was due to haemolysis making it impossible to read titres below 1:20 for most animals.
Antibodies against F. tularensis have been detected in wild animals (red fox, wild boar and raccoon dog) that did not show signs of disease in Austria [Reference Hoflechner-Poltl9], the Czech Republic [Reference Hubalek10] and Germany [Reference Kuehn11, Reference Otto12]. The animal species investigated in these studies, had seroprevalences varying between 6.4 and 7.9% and were considered as good sentinels. The methods used in the different studies were not the same and therefore the numbers are not directly comparable. In our study, the seroprevalence in the investigated wildlife species varied between 2.9 and 10.0% (Table 2). Since the number of examined animals in this study is limited, especially for the European lynx and wolverine, and the sampling was opportunistic, this could not be considered as a true seroprevalence representative for the whole populations. However, the results from this study suggest that all these species produce antibodies, and therefore may be good sentinels for tularaemia. When choosing suitable species for biological indication, the geographical location and the population density of the indicator species in each area should be taken in account.
To confirm the positive results found in screening using a slide agglutination test, a method with higher specificity is needed. One study evaluating an immunochromatographic method in which results are read in 15 min, included immunised or experimentally infected nonhuman primate species, pigs and rabbits with positive test results [Reference Splettstoesser34]. With this type of method titres cannot be established, but it may have an advantage compared to the slide agglutination test because it is easier to read the result. To assess the slide agglutination test some training is needed to learn to see when an agglutination is present, while in the immunochromatographic method a coloured line appears. This is of importance when using the test in field. Enzyme-linked immunosorbent assay (ELISA) tests for antibodies against F. tularensis are available for use on human sera, and ELISA methods have also been used in investigations of wildlife [Reference Al Dahouk35]. A study in wild boar evaluated the effect of haemolysis, testing for antibodies against Suid Herpesvirus 1 [Reference Boadella and Gortázar36]. It was concluded that haemolysis does not affect the test result. ELISA would probably be a suitable method to validate the screening results using the slide agglutination test and would also enable the establishment of antibody titres.
Animals that hunt or scavenge on species prone to develop tularaemia, are at risk to get infected and might perhaps maintain the bacterium in their bodies latently. Since lymph nodes in the region of the throat would be the first to encounter the infective agent upon the oral route of uptake, tonsils and submandibular lymph nodes were sampled in our study. In an Austrian study, F. tularensis was detected in the submandibular lymph nodes in red foxes [Reference Hofer14]. Predators and scavengers harbouring the bacterium might therefore serve as potential reservoirs, but there is also a possibility that they rapidly eliminate the bacteria. In our study, one brown bear, one red fox and one wolf had bacteria in the submandibular lymph nodes and were also detected in the one tonsil suitable for immunohistochemical investigation. Since 14 of the 34 seropositive animals were wild boar, it was unfortunate that no lymphatic tissue was available for these animals.
Active surveillance of sentinel species in endemic areas could provide information on variation in sero-prevalence between years and contributes to the knowledge of disease dynamics. It also enables early indication of spread of tularaemia to new areas. Early indications make it possible to inform hunters and other humans at risk, and the public health service.
In conclusion, brown bears, Eurasian lynx, red foxes, raccoon dogs, wild boar, wolves and wolverines are natural hosts for F. tularensis and may serve as sentinels for tularaemia in Sweden. To elucidate their significance for the disease epidemiology, e.g. as possible reservoirs and/or transmitters, further studies are warranted.
Acknowledgements
We thank Michiel Kroese for performing the microagglutination at WBVR. We thank Tomas Jinnerot, SVA, for performing the PCR analyses. We thank the following projects for providing material to the study: Evaluation of restraining traps 2009–11 and 2013–14, Swedish environmental protection agency. Wild boar – can good management secure the food safety at hunting and slaughter? 2014–15, Swedish University of Agricultural Sciences. The Swedish Raccoon dog project, NV-802-0289-08, NV-03794-15, LIFE09 NAT/SE/000344, Swedish Association for Hunting and Wildlife Management, Swedish University of Agricultural Sciences. SLU, Reproductive patterns and potential among Swedish wild boar (Sus scrofa); Swedish environmental protection agency and the Association for hunting and wildlife management.
Financial support
This work was supported by the Swedish Environmental Protection Agency (Viltvårdsfonden), Grant Number 802-0278-13.
Conflict of interest
None.








