INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is an important cause of bacterial diarrhoea. It is transmitted by faecally contaminated food or water. In industrialized countries ETEC is mainly diagnosed as a cause of travellers' diarrhoea (as reviewed in [Reference Qadri1, Reference Ericsson2]). ETEC is also known to cause foodborne outbreaks [Reference Beatty3–Reference Naimi10], some of which were associated with contaminated produce [Reference Beatty3, Reference Naimi10]. We report a large point-source outbreak of gastrointestinal illness that was caused primarily by ETEC, although some patients had Salmonella enterica serotype Anatum (S. Anatum) infection.
On 14 November 2006 the director of a high school in Greater Copenhagen, Denmark, contacted the regional public health authority to inform them about an outbreak of diarrhoea and vomiting among participants of a school dinner party held on 11 November 2006. Almost all students and teachers of the school (750 people) had attended the party. The same night the first people became sick and by 14 November around 200–300 students and teachers had reported gastroenteritis. The regional food authority (Regional Food Inspection Authority of Eastern Denmark) and the Department of Epidemiology, Statens Serum Institut (SSI) started an investigation in order to identify the vehicle of transmission and initiate appropriate control measures.
METHODS
Epidemiological investigation
To identify the source of infection, a retrospective cohort survey was performed. The cohort was defined as students and teachers, who had attended the party at the high school on 11 November.
A case was a person from the cohort, who presented with diarrhoea (looser stools than normal ⩾3 times in 24 h) or vomiting within 48 h after the meal.
Details about the party arrangements and the menu served were collected through interviews with the school administration and the canteen staff who prepared and served the food.
A questionnaire about date and time of illness onset, type and duration of symptoms, and type and quantity of foods consumed during the party was administered through an internet-based survey system (Defgo-net) [11]. Data on quantity of food consumed was collected in five categories: not eaten, tasted only, eaten <1 portion, eaten 1 portion, and eaten >1 portion for each type of food served. In the analysis, individuals who had not eaten or only tasted a given food item were considered unexposed.
Information about the survey and a link to the questionnaire was circulated to students and teachers via the school's intranet with the request that all who attended the school party on 11 November should complete the questionnaire. The school's intranet was also accessible for ill students or teachers from home.
The statistical analysis was performed using Stata version 8.0 [12]. We computed food specific attack rates (AR), risk ratios (RR), 95% confidence intervals (95% CI) and proportion of cases exposed. For multivariate analysis logistic regression was used.
Microbiological and environmental investigation
Sick party attendees were asked to submit stool samples via their general practitioners to the local clinical microbiology laboratory, where they were cultured for Salmonella, Shigella, Campylobacter, Yersinia enterocolitica [Reference Blom13], Clostridium difficile, and Bacillus cereus. Later on, these samples were tested for diarrhoeagenic Escherichia coli and norovirus at the Danish reference laboratories at SSI. Stool samples from the cooks were investigated at SSI.
A PCR method was used for routine diagnostic identification of virulence genes covering the major diarrhoeagenic E. coli groups [Reference Persson14]: verocytotoxin-producing E. coli (VTEC); enteropathogenic E. coli (EPEC); enterotoxigenic E. coli (ETEC); and enteroinvasive E. coli (EIEC).
ETEC and S. Anatum isolates were typed by pulsed-field gel electrophoresis (PFGE) using the PulseNet USA protocols developed for Shiga-toxigenic E. coli and Salmonella, respectively [Reference Swaminathan15]. The restriction enzyme XbaI was used for both bacteria.
Food Inspectorate, Region East inspected the food catering firm where the dinner had been prepared by professional cooks. Several samples of leftover food from the party (roasted veal with cold red pepper sauce; pasta salad with pesto; mixed salad containing ruccola, beans and tomato) were taken for microbiological investigations. Based on results of these tests, ingredients used for preparation of the pesto were later collected for microbiological investigation.
RESULTS
Epidemiological investigation
During the party, food was served by 28 volunteers (ex-students of the school) and seven kitchen staff. No cases were reported among the volunteers who had served the food, but not eaten it themselves. Volunteers and kitchen staff were not included in the cohort study.
The web-based questionnaire was distributed on 16 November. Within 24 h, 364 responses had been received and by 20 November, out of 750 individuals in the cohort, 435 had completed the questionnaire (response rate 58%); 419 (96·3%) were students.
Among respondents we identified 217 cases (AR 50%). The attack rates in teachers, students of different grades, and by gender were similar (data not shown). In total, 182 cases (84%) became ill within 24 h after the dinner (median incubation period ~18 h) (Fig. 1).

Fig. 1. Distribution of dinner participants with diarrhoea or vomiting (□) by time of symptoms onset (n=253), high school party, Greater Copenhagen, November 2006. ![]() , Cases.
, Cases.
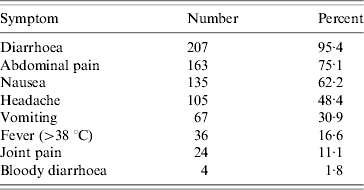
The most frequently reported symptom was diarrhoea (95% of cases), followed by abdominal pain (75%) (Table 1). The diarrhoea-to-vomiting ratio was 3:1. In 49% of cases the duration of diarrhoea was >2 days and in 13% the duration of vomiting was >2 days.
Table 1. Symptoms among cases (n=217) in school party outbreak, Greater Copenhagen, November 2006
The attack rate was higher among participants who had eaten pasta salad with pesto [AR 59·5%, risk ratio (RR) 2·6, 95% CI 1·2–5·7) or bread rolls (AR 56·8%, RR 2·0, 95% CI 1·1–3·4) (Table 2). Ninety-eight percent of the cases had consumed pasta salad and 96% bread rolls. In a multivariate analysis, bread rolls were no longer significantly associated with illness (data not shown). Furthermore, a positive dose–response association was observed for pasta salad with pesto, but not for bread rolls (Table 3).
Table 2. Food specific risk of illness among school party attendants, Greater Copenhagen, November 2006
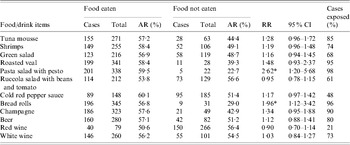
AR, Attack rate; RR, risk ratio; CI, confidence interval.
* P<0·05.
Table 3. Risk of illness among school party attendees according to the amount of different food items consumed, Greater Copenhagen, November 2006
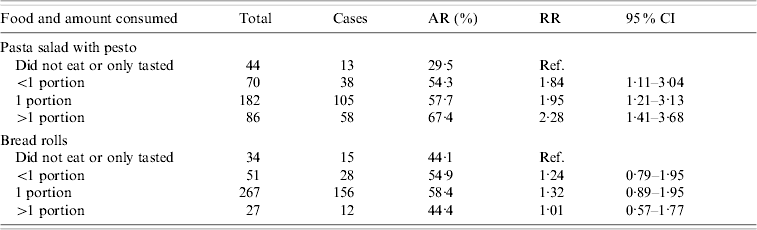
AR, Attack rate; RR, risk ratio; CI, confidence interval.
Microbiological and environmental investigation
A fixed menu consisting of several food items (Table 2) had been served during the party. All but one food item were prepared on the day of the party by four professional cooks. The pasta salad was prepared one day before by a fifth cook, who had also made the pesto for that salad from fresh ingredients a day earlier. None of the cooks reported gastrointestinal symptoms before food preparation or foreign travel in the previous 2 weeks.
Stool samples from 48 persons were examined. Samples of 18 people were positive for ETEC, of which 17 isolates were serotype O92:H− [positive for heat-stable enterotoxin (ST), STh and STp, resistant to ampicillin (AMP), sulphamethoxazole (SMX), streptomycin (STR), trimethoprim (TMP), tetracycline (TET)] and one isolate was serotype O153:H2 (STp positive, resistant to AMP, SMX, STR, TET).
Sixteen of the 17 O92:H− isolates were indistinguishable by PFGE whereas the PFGE profiles of the remaining O92:H− isolate differed from the others by a few bands. The PFGE profile of the O153:H2 isolate was significantly different from the O92:H− isolates.
S. Anatum was found in stool samples of four cases, one of whom was also infected with ETEC. Samples from 30 patients were tested by PCR for norovirus genogroups I and II and were all negative. No enteric pathogens were found in stool samples from the cooks.
Leftovers of the pasta salad with pesto were heavily contaminated with generic E. coli (up to 105 bacteria/g) [16]. E. coli were not detectable (<10 c.f.u/g) in any of the other leftover food items. S. Anatum was also found in the pasta salad. The pesto for the salad had been prepared without heat treatment 2 days before it was served; it was made from fresh basil, pine nuts, garlic, olive oil and Parmesan cheese. Microbiological investigations of all these individual ingredients were all negative for E. coli (<10/g). However, only the pine nuts were from the batch used for the preparation of the pesto in question. The food samples were tested for ETEC by different approaches which included investigations of more than 100 individual presumptive E. coli colonies for presence of genes encoding STp and STh (DNA–DNA polynucleotide probes – dot-blot technique and PCR). In these investigations selective indicative isolation agar, tryptone bile x-glucuronide (TBX) agar was used based on the resistant profile of the outbreak strains. Furthermore the pesto salad was enriched in tryptic soy broth with and without addition of different breakpoint concentrations of antimicrobials. DNA from the enrichment broths were investigated by PCR analysis for presence of STp. All attempts to isolate ETEC were unsuccessful.
S. Anatum isolates from pasta salad and from the four patients were all indistinguishable by PFGE typing. The outbreak strain was different from nine S. Anatum isolates, cultured from animals in Denmark in 2006, and different from the highly diverse collection of 60 S. Anatum isolates present in the PulseNet Europe database.
DISCUSSION
The epidemiological investigation together with the results of the stool samples and food microbiological investigation incriminated a pasta salad with pesto as the most probable vehicle of the outbreak.
The pesto used for the salad had been prepared about 48 h prior to consumption. Based on food microbiological knowledge, the fresh basil seems to be the most likely source of contamination. An alternative culprit could be the pine nuts, but nuts from the same batch as used for the preparation of the dish tested negative for E. coli and were therefore unlikely to be contaminated. None of the other ingredients in the pesto were likely sources. Contamination is believed not to have taken place during food preparation as none of the food handlers had a history of recent illness or foreign travel, none of them were sick when preparing the food, and as all their stool samples tested negative. Furthermore, while a food handler may contaminate a food item with a single pathogen, a multi-agent outbreak including a high concentration of E. coli found in the pesto is more in line with a gross faecal contamination of environmental origin. It is probable that the basil could have been the original source of infection and that bacterial growth, which occurred after the pesto was mixed with a large volume of still lukewarm cooked pasta, contributed to the high degree of contamination.
If the hypothesis of contaminated basil is accepted, how was it then contaminated? The use of wastewater for irrigation is a common practice in many countries. Due to the scarcity of clean and safe water, there are good reasons to use wastewater for agriculture, and also because it provides a cheap and reliable source of nutrients. However, there are clear risks associated with this practice [Reference Katzenelson, Buium and Shuval17–Reference Trang20]. The basil had been imported from Israel, where surface and run-off water is frequently used in irrigation agriculture, albeit restricted to products which are not intended for direct human consumption. Water of drinking water quality must be used for cultivation of fresh produce. We contacted the Ministry of Health concerned, who initiated an inspection of the producer who was declared as the origin of the basil in the importation papers. However, as this producer claimed not to have grown basil in the last 4 years, neither the exact origin of the basil, nor the environmental conditions during production and handling could be clarified. It is possible that the basil was produced by another producer under the brand X (=a false declaration of producer X). As an alternative, the basil might in fact have been produced by producer X, but so denied when the Israeli authorities undertook a local investigation. For example, he might have showed the authorities a site that was used for other types of production only. We do not want to speculate about this, and do not have any knowledge about similar issues in international traceback.
Basil imported from the same country was recently incriminated as a vehicle in a general outbreak of S. Senftenberg primarily in the United Kingdom but also with cases in other countries [21].
The first stool culture results for routine gastrointestinal pathogens (not including diarrhoeagenic E. coli) were negative. This information, combined with preliminary epidemiological data about the short incubation period and a diarrhoea-to-vomiting ratio of around 1:3, suggested ETEC as the possible aetiology of the outbreak. ETEC incubation period in outbreaks can be as short as 10–12 h [22] with a median of 24–48 h and the diarrhoea-to-vomiting ratio is typically >2·5 [Reference Beatty3, Reference Dalton23]. Later, this suspicion was confirmed by detection of ETEC with a single PFGE pattern in a large proportion of stool samples. E. coli in high concentrations and S. Anatum were cultured from leftover pasta salad with pesto, indicating gross faecal contamination. Not surprisingly, it was not possible to detect ETEC in the food samples, because given the high number of E. coli, this would amount to finding the proverbial ‘needle in the haystack’.
The investigation of this outbreak was facilitated by the use of a web-based survey tool, which allowed a rapid dissemination of the questionnaire, collection and analysis of data. The advantages of using the internet in outbreak investigations have been well described previously [Reference Kuusi24, Reference Srikantiah25]. This tool helped to minimize recall bias and facilitated rapid access to the questionnaire by all party attendees, including cases, who stayed at home due to illness. A database with responses was automatically created, thus saving time and avoiding potential errors during manual data entry. A preliminary data analysis, which already indicated the pasta salad as the outbreak vehicle, was available within 24 h of questionnaire distribution. The quantification of the food intake proved to be important, as a fixed menu with relatively few different dishes was served at the party. The establishment of a dose–response relation supported the incrimination of the pasta salad with pesto as the vehicle.
A limitation to our epidemiological investigation was the low response rate of 58%, which might be due to a request for personal identifiers in the questionnaire. We asked for this information to be able to link epidemiological data with the microbiological data. However, we have no indication that the low response rate introduced a relevant bias in the association with the vehicle, because the statistical association of eating pasta salad with pesto was supported by the detection of faecal indicator bacteria in leftovers of this dish. However, the low response rate might have resulted in an overestimated attack rate as those not developing symptoms might be less motivated to respond to the questionnaire.
While this outbreak at the beginning may have looked like a ‘mainstream outbreak’ caused by, e.g. norovirus, both the agents and the vehicle turned out to be unusual, which corroborates the notion that it is always worth the effort to conduct an outbreak investigation. The detection of ETEC as the main aetiology of this outbreak emphasized the need for specific microbiological tests beyond the routine cultures performed in many clinical microbiological laboratories.
The present outbreak is the largest and most thoroughly documented ETEC outbreak in Denmark. Based on the experiences from the present outbreak, we cannot rule out that a proportion of sporadic ETEC infections in Denmark are caused by import of contaminated foodstuffs such as preserved vegetable produce and fresh herbs. To improve food safety further, it is important to target this poorly regulated and researched area. Introducing microbiological quality standards for imported fresh produce should be considered.
ACKNOWLEDGEMENTS
We thank Blenda Böttiger from the Department of Virology, SSI, Charlotte Pers and Lene Nielsen from the Clinical Microbiology Department, Herlev Hospital, and Gitte Sørensen from the National Food Institute, Copenhagen for their contributions to the microbiological investigations. We also acknowledge the cooperation of the Israeli authorities in this investigation.
DECLARATION OF INTEREST
None.






