Introduction
The year 1858 was marked in Martinique by the appearance of an epidemic of jaundice.
O. Saint-Vel, 1862 [Reference Saint-Vel1]So begins Saint-Vel's account of a strange disease that visited the French Caribbean colony. Striking first at St Pierre in April [Reference Ballot2], it then spread across the island, and by the end of the year, left 24 people dead, all women [Reference Saint-Vel1]. At the garrison in nearby Fort-de-France, none of the jaundiced soldiers perished [Reference Decaudin3]. Reflecting on the epidemic's alarming propensity to kill women, Saint-Vel's contemporary in Paris, Hervieux, averred: ‘there was a severe form, always the same, always fatal: the comatose form’ [Reference Hervieux4]. The risk factor for fatality was not just being female, but being pregnant: for that was the condition of 20 of the dead.
The epidemic in Martinique was among the earliest of its kind recorded. Might it have been hepatitis E – a transmissible, jaundice-engendering disease that can not only take on epidemic proportions but also generate excess cases of hepatic coma and death in gestational women? This review examines outbreaks of jaundice that have especially imperilled the pregnant, assessing if those occurrences may be, or are – in fact – hepatitis E.
Literature search
Monographs published in the 19th century which describe diseases that cause epidemics of acute jaundice were consulted for discussion of outbreaks notable for deaths in pregnant women. Reports cited in the key monographs [Reference Decaudin3, Reference Ozanam5–Reference Vinay7] were accessed. The terms ‘jaundice’, ‘yellow atrophy’, ‘liver necrosis’, ‘outbreak’, ‘epidemic’, ‘pregnant’ and ‘women’, in various combinations, were searched among titles, abstracts, or both, in: Tropical Diseases Bulletin; Bulletin of Hygiene; Journal of the American Medical Association including its Current Medical Literature section of volumes 32–144 (1899–1950); The Lancet; British Medical Journal; Transactions of the Royal Society of Hygiene and Tropical Medicine; and the Quarterly Journal of Medicine. These terms were also searched using PubMed. Primary articles thought pertinent were then sourced for review.
Early epidemics
Since the 18th century when epidemics of jaundice started being documented [Reference Ozanam5], two earlier outbreaks were noted for leading pregnant women into coma, then death. One cluster occurred in Ludenscheid in the Palatinate (1794) [Reference Kerksig8] and the other in Roubaix, France (1852–1854) [Reference Carpentier9]. Neither was as devastating as what Saint-Vel witnessed. Two subsequent epidemics in France, which struck Limoges (1859) [Reference Bardinet10] and Paris (1871) [Reference Decaudin3, Reference Hervieux11], wherein fatalities were observed only among gestating (and to a lesser extent, parturient and postpartum) women, alerted continental physicians to the possibility that certain jaundice epidemics could in the course of hitting the population at large put pregnant women at especial risk of death [Reference Vinay7]. A comment by Ollivier (1873) typifies such wonderment:
It is remarkable that in epidemics of jaundice that spontaneously develop in a city or a small region, we see quite often a disease become serious in pregnant women, whereas it was benign in other women [Reference Ollivier13].
Deaths were not confined to the mothers. In Martinique, almost all of the dead women delivered stillborn infants [Reference Saint-Vel1]. Other outbreaks generated high rates of premature delivery, miscarriages and stillbirths not only among the dead but the survivors also. Thus, in Limoges, there were six stillbirths compared to three maternal deaths [Reference Bardinet10]; and in Paris, although of the 16 cases only two died, ten aborted spontaneously or delivered prematurely [Reference Vinay7].
In the Paris outbreak of 1871 [Reference Decaisne12], pregnant case-patients were admitted to La Maternité, the lying-in hospital for the city's poor [Reference Decaudin3, Reference Vinay7, Reference Hervieux11]. Autopsies conducted there to identify the causes of maternal and infant mortality [Reference Fuchs14] attributed the demise of the jaundiced women to acute yellow atrophy. This term had been introduced some three decades before to describe the striking yellow and shrunken appearance at post-mortem of the melting liver, during which it is ‘drowned in the bilious deliquescence, and disappears’ [Reference Wickham Legg6]. Such a vivid morbid entity, correlating microscopically with massive necrosis of the hepatic parenchyma, became associated with a puzzling disorder occasionally encountered in acutely jaundiced patients whereupon coma would supervene, advancing to death, the extent of overt haemorrhage being variable. An over-representation of pregnant women (up to a third) had begun to be noted in case-series studies of patients who died from the condition [Reference Decaudin3, Reference Wickham Legg6, Reference Vinay7] but not until the La Maternité outbreak was it linked directly to fatalities arising from an epidemic.
As the 19th century progressed, four more space–time clusters of jaundice epidemics bearing similar predilections for pregnant women were reported, albeit small in scale compared to the Martinique outbreak. One occurred in Heusenstamm, Germany (1874) [Reference Klingelhoeffer15] and the others in more dispersed localities: St Paul, Minnesota (1874) [Reference Smith16] and an unspecified village in Tennessee (1898) [Reference Young17], the USA; and also Brisbane, in Queensland (1888) [Reference Hardie18]. Only in the Brisbane outbreak were autopsies performed, the livers of four of the five women being found shrunken and rhubarb-yellow. Over the same period in Australia, two other clusters, located in Sydney, were observed in which pregnant women were the most severely affected although none perished [Reference MacDonald19, Reference Creed and Scot-Skirving20].
At the turn of the century, central Italy became the focus of similar epidemics, with clusters appearing successively in Parma (1904–1905) [Reference Bertino21], Portoferraio on the island of Elba (1906) [Reference Queirolo22], Piombino (1908) [23], Porto San Giorgio (1909) [Reference Mancini24] and Soriano nel Cimino (1910) [Reference Cova25]. Simultaneous to the Portoferraio outbreak was an island-wide epidemic which afflicted an estimated 700 inhabitants with jaundice [Reference Queirolo22]. In 1916, another outbreak broke out in the mainland, this time in Galeata [Reference Pignataro26]. At all sites, fatalities were exclusive to pregnant women. Subsequently, except for one notification of a small cluster among natives in Bombay [Reference Tucker27], the flow of publications relating to these mysterious epidemics abated, until about the onset of the Second World War. Passing allusions had been made to jaundice epidemics in Russia prominent for deaths in pregnant women – there was one outbreak that hit Sverdlovsk between 1920 and 1921 [Reference Rabl28, Reference Zhumatov and Dardik29], and another in Tomsk (year of onset unspecified) [Reference Zhumatov and Dardik29] – but count data were not provided. Figure 1 summarizes the epidemics so far described that reported case-frequency data, mortality-frequency data, or both.
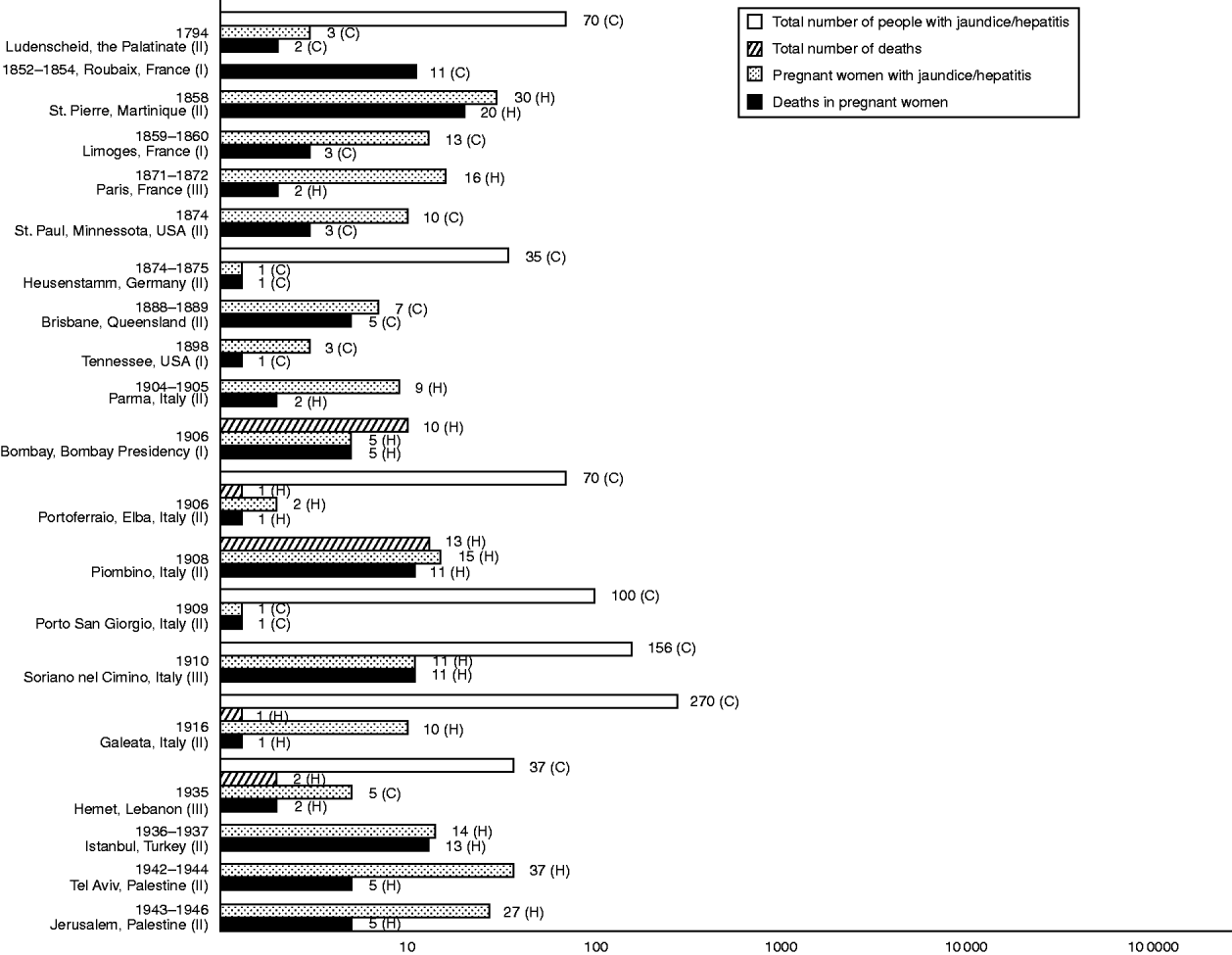
Fig. 1. Morbidity and mortality frequencies in hepatitis E-like epidemics of jaundice or viral hepatitis with pregnancy deaths, 1794–1946. Numerals at end of and alongside bars specify counts. C, Community-based; H, hospital-based. Roman numerals next to names of epidemic location denote class of epidemic according to likelihood of hepatitis E. I, Class I (plausible); II, class II (possible); III, class III (probable). Reference citations: Ludenscheid [Reference Kerksig8]; Roubaix [Reference Carpentier9]; St Pierre [Reference Saint-Vel1]; Limoges [Reference Bardinet10]; Paris [Reference Decaudin3, Reference Vinay7, Reference Hervieux11, Reference Decaisne12]; St Paul [Reference Smith16]; Heusenstamm [Reference Klingelhoeffer15]; Brisbane [Reference Hardie18]; Tennessee [Reference Young17]; Parma [Reference Bertino21]; Bombay [Reference Tucker27]; Portoferraio [Reference Queirolo22]; Piombino [23]; Porto San Giorgio [Reference Mancini24]; Soriano nel Cimino [Reference Cova25]; Galeata [Reference Pignataro26]; Hamet [Reference Yenikomshian and Dennis95]; Istanbul [Reference Kent96, Reference Liepmann97]; Tel Aviv [Reference Soferman101] and Jerusalem [Reference Zondek and Bromberg102].
Nosological and aetiological clarifications
Acute yellow atrophy was initially thought to be a specific disease entity [Reference McDonald30]. Over the early decades of the 20th century it was revealed instead as an outcome of insults to the liver by a variety of infectious agents as well as toxic chemicals, notably phosphorus, chloroform, cinchophen and trinitrotoluene [Reference Willcox31]. Close microscopic studies showed that regardless of cause, degenerative and necrotic changes could in a given hepatic lobule involve hepatocytes surrounding the central vein or the zone between it and the portal vein, or extend to include both zones, even the entire lobule [Reference Opie32] and these changes were variably accompanied by fatty metamorphosis, inflammatory infiltration, stromal collapse, phagocytosis, bile-ductular and hepatocytic regeneration, vascularization and haemorrhage, and fibrosis [Reference Cullinan33]. The form of yellow atrophy that was particularly severe in pregnant women was classified as idiopathic [Reference McDonald30, Reference Cullinan33].
Persuasive evidence implicating idiopathic yellow atrophy as representing the fatal stage of viral hepatitis came to light during the Second World War [Reference Lucké34–Reference Havens and Anderson36] from necropsies performed in American soldiers and their contacts who perished after the soldiers contracted post-vaccinal hepatitis (they had been inoculated with attenuated yellow-fever virus which was cultured and stabilized in contaminated human serum) [Reference Havens and Anderson36]. Acute yellow atrophy, now recognized as having ‘no specific connotation but merely means any acute massive necrosis of the liver’ [Reference Mallory37], was replaced by a more functional, syndromic label that is still current: fulminant hepatic failure (FHF).
The term ‘hepatitis’ presumes inflammatory injury to hepatocytes and implicates the hepatic parenchyma, not mesenchyma, as the primary site of disease. Opposing this concept was ‘catarrhal jaundice’, thought to arise from plugging of the common bile duct by mucus produced from gastroduodenitis. Such notion came to dominate thinking regarding the pathogenesis of simple jaundice (that seemingly distinct form of acute jaundice that was generally self-resolving, not associated with cholelithiasis, and could occur epidemically or sporadically) [Reference Klemperer, Killian and Heyd38, Reference Findlay, MacCallum and Murgatroyd39]. It expanded to accommodate oedematous swelling of the ampulla of Vater and ascending cholangitis as contributing to jaundice. Against this mechanistic interpretation of pathogenesis were findings from prototypic dye-excretion tests and biochemical measurements in blood which pointed to hepatocytic injury [Reference Shattuck, Browne and Preston40]. More critically, whereas autopsy audits showed little or no extra-hepatic bile-duct obstruction [Reference Rich41], microscopic studies using autopsied livers from fatal cases of catarrhal jaundice and cases who happened to die of other causes [Reference Findlay and Dunlop42] and using aspirational liver biopsies conducted on living patients diagnosed with catarrhal jaundice [Reference Axenfeld and Brass43, Reference Dible, McMichael and Sherlock44] presented unequivocal evidence of degenerative pleomorphism among hepatocytes and disruption of the hepatic-cord architecture. Hepatonecrosis was accompanied by lobular, often periportal, infiltration of lymphocytes and other mononuclear cells. Catarrhal jaundice was, without doubt, a hepatitis.
Nor was catarrhal jaundice benign. When it broke out in families [Reference Findlay and Dunlop42, Reference Cockayne45] and among troops [Reference Eppinger46], fatalities could be observed in some cases although most recovered without sequelae. Such disparate outcomes elicited suspicion that acute yellow atrophy and catarrhal jaundice represented extreme ends of a spectrum. Morphological studies verified that the pathology of incipient liver atrophy was not significantly different from that of catarrhal jaundice [Reference Axenfeld and Brass43–Reference Eppinger46], thereby unifying nosologically these two apparently disparate entities.
If the hepatic parenchyma were to be the seat of disease, the causative agent would be one that targets hepatocytes haematogenously from the general circulatory system, or more directly from the portal system. After a spirochaete was discovered as the cause of Weil's disease, extensive investigations were launched to determine if leptospires [Reference Levett47] could be the agent of epidemic catarrhal jaundice: they could not [Reference Findlay, MacCallum and Murgatroyd39]. The typhoid and paratyphoid bacilli were also implicated; any association between infection by these or other enteric bacteria and acute jaundice was later ascribed to intestinal infections and hepatitis co-prevailing under unsanitary conditions [Reference Havens and Wenner48].
During the Second World War the catastrophic epidemics of post-vaccinal hepatitis entangled with outbreaks of camp jaundice that were rife among Allied and Axis forces [Reference Lucké34–Reference Havens and Anderson36, Reference Stokes and Miller49]. These debilitating epidemics galvanized efforts to identify the icterogenic agent. Two forms of hepatitis were confirmed, one form with a short and the other with a long incubation period. The former corresponded with what was being observed in the field as ‘infectious hepatitis’, essentially a filth disease, and the latter with ‘homologous serum hepatitis’, coined to embrace a variety of injection-transmitted diseases other than post-vaccinal jaundice such as post-arsphenamine (salvarsan) jaundice, transfusion jaundice and syringe jaundice [Reference Findlay, MacCallum and Murgatroyd39, Reference Mortimer50]. Significantly, jaundice was transmissible in human serial passage after the volunteers were inoculated with filtered material extracted from serum or stools (for infectious hepatitis), or from predominantly serum (for serum hepatitis), further implicating the icterogenic agent as a virus [Reference McDonald30, Reference Findlay, MacCallum and Murgatroyd39, Reference MacCallum51].
By the time catarrhal jaundice was abandoned from medical parlance [Reference Roy52], it had undergone major appellative transitions. ‘Catarrhal’ was replaced by terms that reflected with increasing precision the mode of disease acquisition or the aetiology; such terms (other than ‘infectious’) were: ‘infective’, ‘common infective’, ‘non-spirochaetal’, ‘epidemic’, and finally ‘virus’ or ‘viral’. The two last-mentioned terms were presumptive, since no viruses had yet been identified. As for ‘jaundice’, it was replaced with ‘hepatitis’, acknowledging that anicteric forms of the disease exist [Reference Sherman and Eichenwald53]. Although infectious hepatitis connoted the short-incubation form that was associated with faecal–oral (or enteric) transmission and recognized in waterborne outbreaks [Reference Mosley54], it could refer generically to both short- and long-incubation forms [Reference Krugman, Giles and Hammond55]. Whether the causative agent comprised strains of one organism or separate organisms [Reference Havens and Anderson36, Reference MacCallum51] was resolved after intensive serological studies and then electron microscopy characterized the agent of the short-incubation disease and that of the long-incubation disease to be distinct viruses, now known as hepatitis A virus (HAV) and hepatitis B virus (HBV), respectively. More years intervened before two other parenterally transmitted viruses, hepatitis C virus (HCV) and hepatitis D virus (HDV), and one other enterically transmitted virus, hepatitis E virus (HEV), were identified [Reference Gust and Feinstone56–Reference Khuroo60].
Attribution of epidemics to hepatitis E
All the outbreaks so far described are more likely than not due to hepatitis E. The defining characteristic of a hepatitis E epidemic is its undue lethality for pregnant women, especially those who have entered the later stages of gestation [Reference Teo61]. Although all the five known agents of viral hepatitis can induce FHF in the infected host, observational studies have shown that in pregnant women, hepatitis A – whether occurring sporadically [Reference Farber62–Reference Ryu65] or epidemically [Reference Ye66, Reference Willner67] – gives rise to no or very low mortality rates, and that deaths from FHF following acute hepatitis B either are absent or occur at significantly marginal rates compared to hepatitis E [Reference Acharya63, Reference Aziz68, Reference Jaiswal69]. As for hepatitis C and D, no tendency for pregnant women to develop FHF after their acquisition has yet been reported [Reference Gonzalez70]. Similarly, non-viral hepatitides that can expand into epidemics of jaundice, such as yellow fever [Reference Monath and Barrett71], leptospirosis [Reference Levett47] and louse-borne relapsing fever [Reference Bryceson72] do not share that predilection. Nor do other diseases for which jaundice could present although less prominently, e.g. typhoid, typhus, dengue and malaria [Reference Syhavong73]. Hepatitis E is therefore pre-eminent among infectious diseases in predisposing pregnant women to FHF [Reference Teo61].
Hepatotoxins, too, can potentially result in community-wide outbreaks of jaundice and liver failure [Reference Meggs74] but none was wont to bring about morbidities in pregnant women. A possible exception is tetracycline which in the 1960s tended to be administered intravenously in large doses to pregnant women with pyelonephritis, a practice that led to reports of excess deaths from liver failure [Reference Combes, Whalley and Adams75]. This antibiotic did not exist when the jaundice epidemics so far described occurred.
A second characteristic of hepatitis E is that during community outbreaks, clinical attack rates are highest among adolescents and young adults than children and older adults [Reference Teo76]. By contrast, when hepatitis A strikes a community, peak clinical attack rates tend to be among young children [Reference Gust and Feinstone56, Reference Jameel77]. A distinction between the two enterically transmitted viral hepatitides may therefore be made. That can be blurred, however, if a jaundice outbreak is associated with HAV and HEV co-transmission [Reference Jameel77–Reference Lay85] or if a hepatitis E outbreak occurs in a locality where HAV infection is highly endemic [Reference Tandon86–Reference Hasan, Omer and Al-Dulami88]. Furthermore, peak attack rates of hepatitis A can shift from children to young adults, but that shift is a relatively recent phenomenon [Reference Tapia-Conyer89, Reference Mathur and Arora90]. Still, other jaundice epidemics have been encountered which although primarily striking adults are associated with neither HAV transmission nor severe disease in pregnant women [Reference Birtaseviæ91–Reference Arankalle94]; they may be caused by yet undiscovered icterogenic agents.
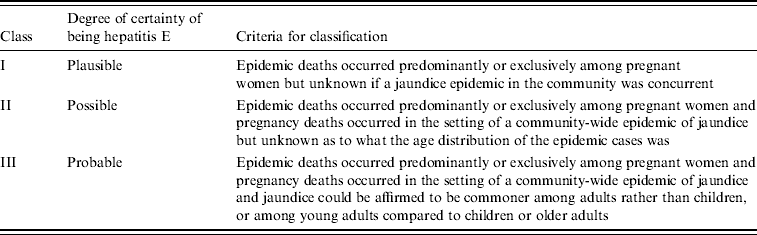
These limitations notwithstanding, a consideration of whether the distinguishing characteristics of hepatitis E, i.e. high attack rates in young adults and deaths in pregnant women, are revealed in historical documentations of jaundice epidemics permits any outbreak to be classified – along a gradation of certainty that the reports pertain to hepatitis E – as belonging to one three classes: class I (plausible), class II (possible), and class III (probable) (Table 1). Each of the epidemics represented in Figures 1–3 has been assigned a class following this classification scheme.
Table 1. Classification of hepatitis E-like epidemics
Epidemic escalations
After an apparent lull between the two world wars, reports of jaundice epidemics that were peculiarly lethal to pregnant women resurfaced. A change in trend to these outbreaks is perceptible: they were being reported more frequently; the venues were shifting eastward and southward; and their scale grew. All the outbreaks are readily assignable to class II or class III. Heralding this new era was a small cluster in 1935 in Hemet, Lebanon, which led to two fatalities, both in pregnant women [Reference Yenikomshian and Dennis95]. A year later and over a period of 16 months, all but one of 14 jaundiced pregnant women who were admitted with coma to the university hospital in Istanbul, Turkey, died (Fig. 1) [Reference Kent96]. Attending obstetricians were, like their European predecessors, mystified by the exclusivity of deaths among pregnant women. As Liepmann (1938) recounted:
But the strangest thing to our epidemic is, that in a time of widespread jaundice throughout the city and in all hospitals in Istanbul, only the pregnant women were affected by acute yellow atrophy, and non-pregnant women and men did not suffer it [Reference Liepmann97].
In Palestine, 1941, the first of a succession of hepatitis epidemics erupted, coinciding with the onset of mass immigration into the region. It broke out in detention camps and involved mainly young adults [Reference Kligler, Btesh and Koch98]. Pregnant women were noted to bear the brunt of severe hepatic disease and of deaths from acute yellow atrophy [Reference Leffkovitz99, Reference Btesh100] but count data were not reported. More indicative of hepatitis E was an outbreak that followed which generated steep increases in the number of jaundiced pregnant women admitted to hospitals in Tel-Aviv between 1942 and 1944, and in Jerusalem between 1944 and 1946, with case-fatality rates (CFRs) of 14% and 19%, respectively [Reference Soferman101, Reference Zondek and Bromberg102] (Fig. 1). Upsurges in immigration after the founding of Israel brought about even more dramatic rises in the frequency and extent of hepatitis epidemics [Reference Davies and Suchowolski103]. A notable outbreak that smouldered in the north in the 1950s led to excess hospital admissions of jaundiced pregnant women in Haifa, the CFR being about 9% [Reference Peretz, Paldi and Barzilai104] (Fig. 2). Predating it was a series from neighbouring Jezreel and Afula involving 55 cases observed sporadically over 10 years of which nine cases were fatal [Reference Asher105]. Notifications in Israel of such sporadic and outbreak occurrences subsequently ceased [Reference Shalev and Bassan106].
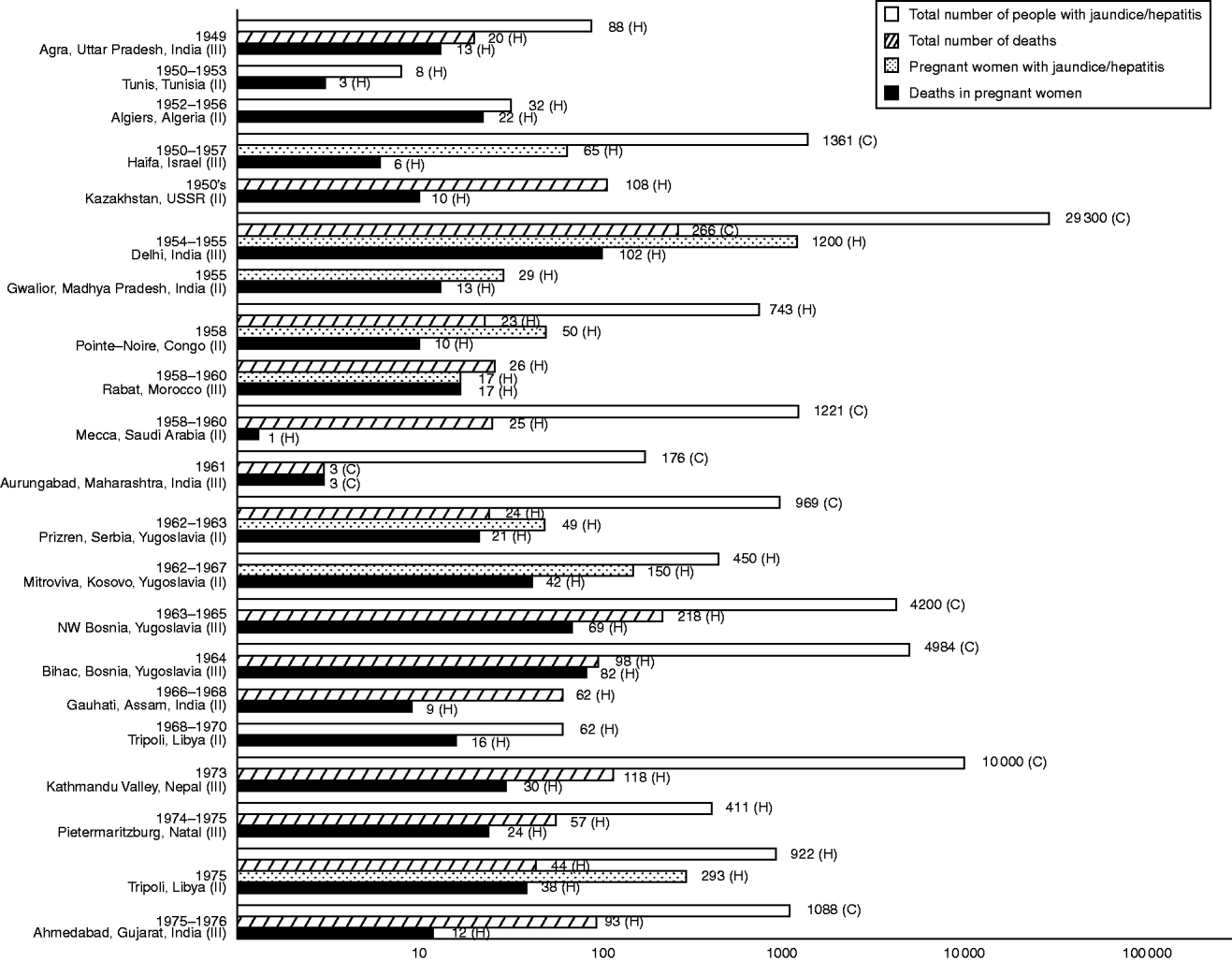
Fig. 2. Morbidity and mortality frequencies in hepatitis E-like epidemics of jaundice or viral hepatitis with pregnancy deaths, 1949–1976. Reference citations: Agra [Reference Wahi and Arora150]; Tunis [Reference Corcos108]; Algiers [Reference Bourdon and Ezes109]; Haifa [Reference Peretz, Paldi and Barzilai104]; Kazakhstan [Reference Zhumatov and Dardik29]; Delhi [Reference Viswanathan151]; Gwalior [Reference Phatak and Patil155]; Pointe-Noire [Reference Combescot de Marsaguet and Thomas133]; Rabat [Reference Bablet131]; Mecca [Reference Hammouda262]; Aurungabad [Reference Dhamdhere and Nadkarni156]; Prizren [Reference Perisić145]; Mitrovica [Reference Colaković, Dragović and Vućelić146]; NW Bosnia [Reference Kallai, Gaon and Pinjo147]; Bihac [Reference Birtasević148]; Gauhati [Reference Das157]; Tripoli, 1968–1970 [Reference Wyatt112]; Kathmandu Valley [Reference Hillis, Shrestha and Saha159]; Pietermaritzburg [Reference Parkes144]; Tripoli, 1975 [Reference Christie113] and Ahmedabad [Reference Sreenivasan158].
Along the southern Mediterranean littoral arose a succession of reports of fatal jaundice in pregnant women: first from Tunisia (from 1945) [Reference Corcos107, Reference Corcos108] thereafter Algeria (from 1952) [Reference Bourdon and Ezes109, Reference Houel, Fabregoule, Gares and Bourdon110] and then Morocco (from 1958) [Reference Hugonot111] (Fig. 1). Somewhat later, in 1968 and 1970, similar reports came from Libya [Reference Wyatt112, Reference Christie113]. In all these, jaundice in the wider community was also noted, but deaths in males and non-pregnant women were seldom observed. Features common to the illness suffered by pregnant women were: previous paucity of occurrences as perceived by the reporting physicians; onset during the third trimester; susceptibility of both native and expatriate women; fulminant course leading up to encephalopathy; high rates of fetal loss; and pathological findings redolent of classic acute yellow atrophy. Light microscopy of post-mortem or biopsy liver samples revealed frank hepatocytic necrosis, which was distinct from appearances associated with gestational conditions affecting the liver that could occasionally be fatal such as hyperemesis gravidarum, eclampsia and acute fatty liver of pregnancy [Reference Sheehan114]. These various features are consistent with hepatitis E being newly introduced to the region. By the end of the second decade following the Second World War, endemicity was firmly established: subsequent reports would relate to sporadic disease mainly [Reference Delons115–Reference Ghazli121], whereas those related to outbreaks became occasional [Reference Gebreel and Dane118, Reference Belabbes122, Reference Benjelloun123].
The aforementioned class II or III epidemics struck the Maghreb [Reference Corcos107–Reference Christie113] when epidemics of jaundice were breaking out among soldiers [Reference Merveille and Heuls124, Reference Petchot-Bacque125], mostly deployed from French Equatorial Africa [Reference Lemaire and Deroo126]. As the magnitude of the outbreaks and the extent of fulminant disease and cirrhosis among the military cases varied [Reference Merveille and Heuls124, Reference Petchot-Bacque125, Reference Gaubert127] and because no women were involved, it is not possible to assess the contribution of hepatitis E. Confounding that difficulty was the encroaching endemicity of parenterally transmitted hepatitis in the African continent, associated with mass vaccinations, widespread availability of syringe-mediated treatment for diseases such as yaws, syphilis and schistosomiasis, and of myriad other injection-based therapies including blood transfusions and plasma infusions administered to civilian and military populations [Reference Findlay, Kirk and Lewis128–Reference Frank130]. The natural histories of the new forms of viral hepatitis were often grave [Reference Findlay, Kirk and Lewis128, Reference Bablet131] which contrasted with the essentially benign nature of epidemic catarrhal jaundice (insofar as it could be distinguished from leptospirosis, yellow fever and relapsing fever) that had episodically been reported from Africa since the First World War [Reference Beheyt129, Reference Kirk132]. Whether the higher morbidity and mortality rates were linked to dissemination of new and more virulent strains of HBV or HCV, or to HDV super- or co-infection of people with persistent HBV infection remains uncertain [Reference Mortimer50]. Nonetheless, a class II hepatitis E-like epidemic in 1958 was reported from Belgian Congo [Reference Combescot de Marsaguet and Thomas133], probably a massive one as >700 cases of jaundice were hospitalized (Fig. 1).
The striking observations reported from northern Africa prompted retrospective observational studies in Dakar, Senegal [Reference Mazaud, Moissincac and Labegorre134, Reference Payet135]. A major investigation of >400 jaundiced patients admitted to a single hospital between 1954 and 1959 found a CFR from acute hepatic failure of 24% in pregnant women, contrasting with 11% in non-pregnant women [Reference Payet135]. These findings are suggestive of hepatitis E, but in an endemic setting, as no community epidemics of jaundice were being reported. The situation is mirrored in Accra, Ghana, where over a 1-year period in 1963, and in the absence of a concurrent epidemic, hospitalized cases of presumptive viral hepatitis were studied; the clinical attack rate and the incidence rate of coma were both four times higher in pregnant than non-pregnant women [Reference Morrow136]. From Nigeria was reported a 1960 epidemic of jaundice that particularly afflicted pregnant women [Reference Morrow136, Reference Ogunlesi137] but further information is unavailable. Near-contemporaneous, autopsy-based audits conducted in Ibadan revealed massive hepatic necrosis to be a significant cause of maternal mortality, accounting for 20% of non-obstetric deaths in a 1957–1960 series [Reference Lawson138] and 8% of all deaths in a 1961–1968 series [Reference Ojo, Francis and Onifade139].
Reports of epidemics during the immediate decades after the Second World War in eastern and southern Africa did not mention deaths in pregnant women [Reference Beheyt129, Reference Kirk132, Reference Sood, Hulley and Hutt140] nor did those of studies into sporadic viral hepatitis [Reference Jindani, Bagshawe and Forrester141, Reference Moffat and Gelfand142]. An audit of viral hepatitis in pregnancy observed between 1957 and 1967 in a Johannesburg hospital uncovered only 12 cases, none fatal [Reference Hurwitz143]. Then, from 1974 to 1975, a steep rise was observed in the number of jaundiced women admitted to the obstetric wards in Pietermaritzburg [Reference Parkes144]. The CFR was 44%, and all the deaths followed hepatic coma (Fig. 2). Admissions of jaundiced patients to the medical wards showed a corresponding rise, pointing to a class III epidemic in the environs.
Meanwhile, a spate of class II and III epidemics befell several republics and provinces of what was then Yugoslavia. Occurring in the early 1960s and centered on Prizren (in Serbia) [Reference Perisić145], Mitrovica (in Kosovo) [Reference Colaković, Dragović and Vućelić146], an unidentified town in Bosnia [Reference Kallai, Gaon and Pinjo147], and Bihac (also in Bosnia) [Reference Birtasević148], they generated hospital-based CFRs in pregnant women that were unusually high, ranging from 28% to 43% (Fig. 2). Cases and deaths were almost exclusive to the poorer, Muslim communities. Representing the last of their kind in Europe, these epidemics appeared during a period when numerous waterborne outbreaks of jaundice erupted across the federation [Reference Birtasević149].
Elsewhere, other jaundice epidemics associated with pregnancy deaths were being reported. In the Indian subcontinent there was a 1949 cluster of in-patient cases outstanding for its 65% CFR among pregnant women [Reference Wahi and Arora150] (Fig. 2). It arose from an epidemic, whose magnitude is unknown, which affected villages around Agra. Six years later, a much larger outbreak hit Delhi: within 8 weeks, >250 people were dead, with almost half of them pregnant [Reference Viswanathan151] (Fig. 2). Of 1200 pregnant women notified as cases, the obstetric history of 339 was known, and of these, 101 delivered stillbirths or infants who died as neonates [Reference Naidu and Viswanathan152]. Before the Agra and Delhi outbreaks, jaundice epidemics were already being reported; nevertheless, none except the 1906 cluster (27) (Fig. 1) was recorded to have particularly killed pregnant women [Reference Roy52, Reference Raman153, Reference Patel154]. After the Delhi outbreak, more reports of class III epidemics ensued. Most affected relatively smaller conurbations [Reference Phatak and Patil155–Reference Sreenivasan158] but in 1973, an epidemic swept over the Kathmandu valley in Nepal [Reference Hillis, Shrestha and Saha159] (Fig. 2). Shrestha & Maila, who had to face up to en masse hospital admissions that resulted, wrote a moving account of toiling pregnant women being felled by hepatitis E gone rampant:
Twenty-six were brought in coma. They gave history of jaundice of short duration of 2 to 7 days, but were still working till they suddenly became drowsy and comatose and brought to hospital. Of these, 3 were not aware of the disease and were still working the field till they were suddenly struck by hepatic encephalopathy [Reference Shrestha and Maila160].
The abrupt onset of FHF and its fatal outcome likely attenuate the accuracy by which CFRs relating to hepatitis E in pregnant women are calculated. Those who perish without having been admitted to the hospital or otherwise become notified would not have been counted. Also influencing the derivation of the CFR numerator is how the gestational status of the deceased is ascertained: whereas cases who are visibly pregnant would be identified, those in early pregnancy might not. The epidemic scale and intensity bear on both the numerator and denominator. Larger epidemics tend to mandate sample field-surveys, the morbidity and mortality data collected being then used to estimate the CFR [Reference Viswanathan151, Reference Dhamdhere and Nadkarni156, Reference Sreenivasan158, Reference Khuroo161–166]. Such crude estimations would be unnecessary for small outbreaks since cases and deaths could be counted more closely or notified more directly. Finally, CFRs with denominators based on morbidity of community cases are probably lower than those based on morbidity of hospital inpatients as the latter would tend to capture the severer cases. To aid comparative assessments of CFRs, morbidity and mortality counts in Figures 1–4 are denoted according to whether they originated from the hospital (H) or the community (C).
Widening epidemic contexts
Retrospective serological testing for HAV and HBV in patients who developed jaundice during waterborne epidemics in India [Reference Wong167] revealed that they contracted what came to be known as enterically transmitted non-A, non-B (ET-NANB) hepatitis [Reference Reyes, Bradley and Lovett58]. ET-NANB hepatitis was, in turn, determined by later serological testing for anti-HEV antibodies to be mostly hepatitis E [Reference Arankalle94, Reference Khuroo168–Reference Prakash170]. The Indian subcontinent has continued to host many epidemics of ET-NANB hepatitis and hepatitis E. Outbreaks have erupted across India, Nepal, Burma, Pakistan and Bangladesh, in both urban [Reference Jameel77, Reference Myint78, Reference Sailaja84, Reference Tandon86, Reference Das157, Reference Sreenivasan158, Reference Sreenivasan162, Reference Naik164, 166, Reference Khuroo171–Reference Rab176] and rural [Reference Deo and Autkar174, 177–Reference Dilawari180] settings (Figs 2–4). HEV strains associated with these outbreaks, whenever characterized, belonged to genotype 1, there being altogether four genotypes (1–4) that infect humans [Reference Arankalle181]. A very severe epidemic affected about 200 villages in the Kashmir valley in 1978 and 1979. It led to an estimated 600 deaths, two-thirds in pregnant women [Reference Khuroo161, Reference Khuroo168, Reference Khuroo171]. Some cases manifested a cholestatic form of disease similar to that in pregnant women with FHF in Dakar, Delhi and Accra [Reference Payet135, Reference Gupta and Smetana182, Reference Morrow183]. A larger epidemic visited the city of Kanpur during 1992 [Reference Naik164]. The CFR for pregnant women there (27%) was reportedly less than in Kashmir (73%), a disparity ascribable to mortality data for Kanpur having been based on hospital-inpatient counts and those for Kashmir projected from data obtained during a sample survey [Reference Khuroo161, Reference Khuroo171]. Nonetheless, the possibility is raised that outbreaks were caused by HEV strains of varying virulence. Indeed, a few confirmed hepatitis E outbreaks have been documented for which deaths when inquired into were absent [Reference Bhagyalaxmi, Gadhvi and Bhavsar184] or not found among pregnant women [Reference Bali185–Reference Swain188].
Outbreaks apart, numerous case-series have been published [Reference Acharya63, Reference Aziz68, Reference Jaiswal69, Reference Bhasker Rao and Ganapathy189–Reference Hossain215] as well as autopsy reviews [Reference Deodhar216] and audits [Reference Konar217, Reference Shrestha, Saha and Karki218] linking viral hepatitis, ET-NANB hepatitis and hepatitis E with pronounced maternal mortality rates. Fetal complications have also been frequent [Reference Khuroo and Kamili206, Reference Kumar207, Reference Bista and Rana209–Reference Patra211, Reference Hossain215]. More recent, verbally reported autopsy data from population-based studies in Bangladesh reveal 20% of all maternal deaths and 10% of all neonatal deaths to be associated with jaundice in pregnant women, HEV being the chief suspect [Reference Gurley219]. Collectively, these reports indicate that for the last half century, hepatitis E in south Asia – whether epidemic or sporadic – has imposed enormous morbidity and mortality burdens on pregnant women and their infants in excess of that on the general population [Reference Clark220, Reference Labrique221].
Reports from Central Asia of epidemic hepatitis, also called Botkin's disease [Reference Tarejev222] were common in the immediate decades after the Second World War. Many waterborne epidemics were likely hepatitis A in view of the high clinical attack rates in children [Reference Tarejev222–Reference Diachenko225]. Association with fatal FHF in pregnant women was specifically documented for two outbreaks: one outbreak (magnitude unknown) struck an agricultural area in southern Kazakhstan during the 1950s and elicited a CFR in hospitalized pregnant women of 10% [Reference Zhumatov and Dardik29] (Fig. 1); the other was in Kirgizstan between 1956 and 1957 with a CFR of 16% [Reference Farber62]. Over this period, CFRs reported for pregnant women with sporadic Botkin's disease in Central Asia (about 4%) [Reference Farber62, Reference Zakirov226] were similar to those in Russia (between 1% and 6%) [Reference Farber62, 227–Reference Romanov231]. Towards the end of the 1980s a succession of class III ET-NANB hepatitis epidemics struck Central Asia. These hit: Tashauz province, Turkmenistan (1984, and then 1985) [Reference Favorov232–Reference Albetkova234]; southern Uzbekistan (1986) [Reference Favorov79, Reference Sharapov235]; the city of Leninabad, Tajikistan (1986) [Reference Rafiev83, Reference Iarasheva236]; eastern Uzbekistan (1987) [Reference Sharapov235]; and Inner Tien Shan, Kirgizstan (1987) [Reference Usmanov93] (Fig. 3). Typically autumnal in onset, lasting until winter and each affecting between 10 000 and 30 000 people [Reference Favorov237], they were particularly lethal to pregnant women, the CFRs (as estimated from sample surveys) ranging from 7% to 27%. Later testing of sera sampled from the cases confirmed that all the epidemics were hepatitis E [Reference Usmanov93, Reference Albetkova234, Reference Sharapov235, Reference Favorov237] and caused by HEV genotype 1 [Reference Favorov237]. Thereafter, except for a few scattered outbreaks [Reference Rafiev83, Reference Temper, Moroz and Chernysheva227], large-scale epidemics abated. To date, sporadic hepatitis E continues to be observed [Reference Rafiev83, Reference Sharapov235, Reference Iarasheva238].

Fig. 3. Morbidity and mortality frequencies in epidemics of hepatitis E-like, ET-NANB hepatitis with pregnancy deaths, 1978–1990. Kashmir Valley [Reference Khuroo161, Reference Khuroo168, Reference Khuroo171]; Azamgarh [Reference Tandon86]; Calcutta [Reference Pain, Chakraborty and Choudhury172]; Vidisha [Reference Chakraborty163]; Medea [Reference Belabbes122]; Kolhapur [Reference Sreenivasan162]; Rangoon [Reference Myint78]; Kathmandu Valley [Reference Kane178]; Tortiya [Reference Sarthou280]; Rundu [Reference Isaäcson278]; Tashauz [Reference Favorov232–Reference Albetkova234]; S Uzbekistan [Reference Myint78, Reference Sharapov235]; Maun [Reference Mast, Kishioka, Suzuki, Mishiro and Oda285]; refugee camps, Somalia [284]; refugee camps, Sudan [284]; Karachi [Reference Smego and Khaliq173]; S. Xinjiang [Reference Cao239]; Sulangong [Reference Ji241] and Buldana [Reference Deo and Autkar174].
Attendant to the Tien Shan outbreak [Reference Usmanov93] were two consecutive class III ET-NANB epidemics that blighted neighbouring Xinjiang, China, in the autumns of 1987 and 1988. Together, they afflicted nearly 120 000 people (mostly Uighurs) in 23 counties and towns across three prefectures [Reference Cao239], yielding a CFR for pregnant women of 13% (Fig. 3). These occurrences also were later confirmed to be hepatitis E and caused by HEV genotype 1 [Reference Huang240]. Similar, but lesser-intensity outbreaks had earlier [Reference Ji241] and since [Reference Xia242] struck Xinjiang. In the rest of China and in Mongolia, prior ET-NANB hepatitis outbreaks had been described [Reference Zhuang, Wen, Xu and Melnick243] although details are unavailable. Even earlier documentations from China revealed extensive jaundice epidemics in the 1920s but pregnant women were not noted to be especially ill [Reference Wyatt244, Reference Robinson245]. A jaundice-in-pregnancy series from Taiwan covering cases observed from 1961 to 1974 found a 14% CFR associated with viral hepatitis [Reference Soong246]. An investigation in 1962 of Botkin's disease in pregnant women based in Ulan Bator reported a strikingly high CFR of 81% [Reference Gamma247]; whether this was generated in the course of an outbreak is unclear. Serologically confirmed HEV infection is now infrequent in Mongolia [Reference Tsatsralt-Od248]. In China, however, sporadic HEV infection and FHF still occur in pregnant women [Reference Zhu249–Reference Li251]. A shift in HEV endemicity from genotype 1 to genotype 4 (and to a lesser extent, to genotype 3) is being observed [Reference Zhang252] so a decline in the rates of sporadic hepatitis E-related FHF in pregnant women may follow; genotypes 3 and 4, unlike genotype 1, are not closely associated with FHF in younger adults [Reference Teo61].
Four ET-NANB hepatitis or hepatitis E outbreaks have so far been reported from Southeast Asia: two which hit successively one district in West Kalimantan, Indonesia (1987 and 1991) [Reference Lubis253, 254]; one in southwestern Vietnam (1994) [Reference Corwin255]; and most recently one in Java, Indonesia (1998) [Reference Sedyaningsih-Mamahit256]. All the epidemic sites were riverine villages. Death rates were pronounced among pregnant women in the Kalimantan epidemics, the CFR calculated from the 1991 outbreak being 26% (Fig. 4). They were not encountered in the Vietnamese and Javan outbreaks, although surveillance for post-jaundice outcomes in pregnant women was in place. For the rest of Southeast Asia and in East Asia, sporadic hepatitis E is being reported but not severe disease in pregnant women [Reference Syhavong73, Reference Corwin257–Reference Yano261]. In Japan, hepatitis E-associated FHF has been observed; peculiarly, it afflicts predominantly older people, reflecting the endemicity of HEV genotypes 3 and 4 rather than genotype 1 [Reference Teo76].

Fig. 4. Morbidity and mortality frequencies in serologically-confirmed hepatitis E epidemics with pregnancy deaths, 1987–2010. Kathmandu Valley [Reference Shrestha179]; Jammu [Reference Khuroo168]; Kamal [Reference Dilawari180]; Lower Shibeli region [Reference Bile277]; Sintang [Reference Lubis253]; Liboi [Reference Mast, Kishioka, Suzuki, Mishiro and Oda285]; Kanpur [Reference Naik164]; Djibouti [Reference Coursaget81]; Islamabad [Reference Rab176]; Bangui [Reference Goumba, Konamna and Komas282]; Darfur [Reference Boccia165]; Madi Opei and Palonga [Reference Teshale286]; Tongi [166] and Rajshahi [177].
In Mecca during 1958–1960, upsurges in admissions for jaundice to hospitals pointed to an ongoing class II outbreak but it was relatively benign (Fig. 2) [Reference Hammouda262]. Case-series studies of sporadic viral hepatitis conducted in other regions in the Middle East revealed variable rates of FHF and ensuing mortality in pregnant women [Reference Borhanmanesh263–Reference Medhat266]. Given the lack of specific diagnostic testing, it cannot be excluded that such variation in rates reflects regional differences in endemicity of the hepatitis viruses. A more recent, prospective study of HEV infection in the United Arab Emirates found that 21% of women screened antenatally to be viraemic developed FHF as pregnancy progressed [Reference Kumar267] specifically associating HEV with detrimental maternal outcomes.
Reports of localized ET-NANB hepatitis epidemics from Mexico [Reference Velázquez87] (caused by HEV genotype 2 [Reference Huang268]) and similar-scale but more frequent hepatitis E outbreaks from Cuba [Reference Lay85, Reference Montalvo Villalba269] (caused by genotype 1) have not noted fatalities in pregnant women. Earlier reports of sporadic viral hepatitis in pregnant women from Brazil [Reference Shiroma270] and Mexico [Reference Reyes, Saldana García and Valenzuela271, Reference Díaz Saldaña272] showed deaths from hepatic failure, but without specific diagnostic testing attribution to hepatitis E is tenuous. Although sporadic hepatitis E has since been identified in Central and South America [Reference Lay85, Reference Lyra273–Reference Ibarra275], there is little evidence to link it with severe maternal illness [Reference Reyes276].
Africa has continued to provide settings from which class III ET-NANB hepatitis and hepatitis E epidemics frequently break out. The largest outbreak lasted from 1988 to 1989 and affected >140 villages along the Shebeli River in Somalia [Reference Bile277]. Mortalities in the general and pregnant populations – however incompletely determined given the widespread and protracted nature of the epidemic – were appreciable (Fig. 4). Other outbreaks reported are clustered around urban centers: Medea, Algeria (1980–1981) [Reference Belabbes122]; Rundu, Namibia (1983 [Reference Isaäcson278] and then 1995 [Reference Maila, Bowyer and Swanepoel279]); Tortiya, Côte d'Ivoire (1982–1984) [Reference Sarthou280]; Maun, Botswana (1985) [Reference Byskov281]; Djibouti (1993) [Reference Coursaget81] and Bangui, Central African Republic (2002) [Reference Goumba, Konamna and Komas282]. Although relatively circumscribed and brief, FHF-associated fatalities in pregnant women were noted (Figs 3 and 4). For the 1995 Namibian outbreak no pregnant women died although FHF was observed [Reference Maila, Bowyer and Swanepoel279]. The transmitting HEV was identified as genotype 2 [Reference He283] contrasting with genotype 1 that was associated with the earlier outbreak [Reference Isaäcson278]. Refugee camps have been another, more recent epidemic setting. Outbreaks have occurred among displaced people held in camps situated in Somalia [284], Kenya [Reference Mast, Kishioka, Suzuki, Mishiro and Oda285], Sudan [Reference Boccia165, 284] and Uganda [Reference Teshale286]. Owing to the dense concentration of people being accommodated, the outbreaks were larger than those in the cities and towns [Reference Coursaget81, Reference Belabbes122, Reference Isaäcson278–Reference Goumba, Konamna and Komas282]; correspondingly, death counts in pregnant women were higher (Figs 3 and 4). HEV infection remains endemic across much of Africa, as exemplified by findings from the many seroincidence studies conducted [Reference Belabbes122, Reference Rioche287–Reference Adjei293]. Sporadic transmissions still pose fatal risk to pregnant women [Reference Belabbes122, Reference Mirghani, Saeed and Basama294–Reference Hannachi299].
Implications
The narrative as assembled here dates back to the closing years of the 18th century, and traces the shift of sites of epidemic jaundice from Western Europe, the Caribbean, USA and Australia to Eastern Europe, Central and South Asia, and Africa. Accompanying that widening geographical reach is an increasing frequency and magnitude of the outbreaks. This pattern of dispersal could plausibly be an artifact of Western colonization, as outbreaks were reported by Western observers inquiring into diseases first at home, then in the colonies as territories were being possessed [Reference Guerra300, Reference Chernin301], and thereafter – during the post-colonial period – reported by native observers. Whether from expatriate or indigenous reporting, the paucity of pregnancy deaths in documentations of jaundice epidemics from South Asia and Africa during the pre-Second World War 20th century [Reference Roy52, Reference Beheyt129, Reference Bablet131, Reference Kirk132, Reference Raman153, Reference Patel154] is striking. Nonetheless, the possibility that there were outbreaks that went unnoticed or unrecorded cannot be excluded. Gaps in the narrative may also be due to limitations in the reach of this literature review, and missing or inaccessible records [Reference Rabl28, Reference Zhumatov and Dardik29, Reference Myint78, Reference Ogunlesi137, Reference Zhuang, Wen, Xu and Melnick243].
Other than an artifact of reporting, the epidemic history of hepatitis E here constructed and the pattern of diffusion inferred could reflect the manner by which the virulence of HEV for humans has changed over the last two centuries. All serologically confirmed hepatitis E outbreaks associated with pregnancy deaths (Fig. 4) are caused by HEV strains belonging to genotype 1, not the other genotypes. The class I, II and III and the ET-NANB hepatitis epidemics (Figs 1–3) may be presumed to be caused by HEV genotype 1 too. The time of appearance of the pre-20th century class I and II epidemics (Fig. 1) coincides with the time when HEV genotype 1 emerged from its putative immediate ancestor about 100–200 years ago, as estimated by recent molecular-clock analyses [Reference Purdy and Khudyakov302]. Moreover, the dramatic increase in the frequency and scale of post-Second World War class II and III, ET-NANB hepatitis and hepatitis E epidemics (Figs 1–4) corresponds to a period when there was a burst of genetic diversity in genotype 1 strains. How these strains lead to severe hepatic disease in the pregnant remains unknown [Reference Teo61]. Although studies of HEV-infected pregnant women in India have found higher levels of oestrogen, progesterone and β-human choriogonadotrophin in the blood [Reference Jilani303] and higher DNA-binding activity of NF-κB in peripheral blood mononuclear cells and liver [Reference Prusty304] of patients with FHF compared to those without FHF, the manner by which host factors interact with HEV in influencing the pathogenesis of FHF requires clarification.
The narrative also is one of tragedy in maternal and child health continuing into modern times, particularly for South Asia and Africa. To avert that tragedy requires universalizing access to drinking water and basic sanitation [305], in itself an arduous undertaking [Reference Hutton and Bartram306]. Necessarily complementing efforts to meet this challenge is the implementation of measures that are directed specifically to protect against or mitigate the pernicious outcome of HEV infection in pregnant women. Providing these women with antiviral prophylaxis during an epidemic is not yet an option.
Vaccination is a more pre-emptive and now an achievable means to prophylaxis. Two vaccines have completed clinical and safety efficacy trials [Reference Zhu249, Reference Shrestha307]. Whereas the development of one vaccine [Reference Shrestha307] has stopped [Reference Basnyat308], the production of the other vaccine [Reference Zhu249] is progressing and market approval is being sought. Sufficient quantities could soon be available to immunize communities where epidemics have arisen and HEV genotype 1 is endemic. It would then be possible to determine its efficacy against HEV infection and FHF in vaccinees who are or subsequently become pregnant, along with adverse obstetric and fetal outcomes that may ensue.
ACKNOWLEDGEMENTS
The author thanks: Z. E. Dimitrova for generation of the figures; T. Al-Hadithi, R. Aggarwal, M. Favorov, J. A. Frean, E. S. Gurley, Y. J. Hutin, S. Kamili, M. Khuroo, R. Larasati, E. E. Mast, P. P. Mortimer, M. A. Purdy, T. Rachmawati, R. Swanepoel, N. S. Xia and H. Zhuang for helpful discussions; J. Drobeniuc, L. M. Ganova-Raeva, Y. Khudyakov, A. P. Kourtis, L. Ruggiero, E. C. J. Teo, N. Usmanova, G. Vaughan and G. Xia for assistance with translation; and staff from the Public Health Library and Information of the Centers for Disease Control and Prevention (CDC), Biblioteca Polo San Paolo, Biblioteca Unificata Area Medica ‘Adolfo Ferrata’, the Resource Center of the American College of Obstetricians and Gynecologists, the Library of the Royal College of Obstetrics and Gynaecologists, and the Wellcome Library for facilitating access to documents. The findings and opinions in this review are the author's and do not represent the official position of the CDC.
DECLARATION OF INTEREST
None.







