INTRODUCTION
In the EU, yersiniosis is the third most common zoonotic foodborne disease causing 2·6 human infections/100 000 population [Reference Nørrung and Buncic1]. In Finland, the annual incidence rate of yersiniosis infection is higher than the EU average at 11 cases/100 000 population [2] with considerable regional variation existing in the number of cases within the country. Yersinia enterocolitica causes acute gastroenteritis with fever and occasionally bloody, watery diarrhoea, especially in children. Pseudoappendicitis is a common clinical syndrome in young adults and secondary immunological reactions may lead to reactive arthritis and erythema nodosum [Reference Bottone3].
Pathogenic Y. enterocolitica is frequently isolated from the intestinal contents and tonsils of healthy pigs which are considered to be the major reservoir of Y. enterocolitica [Reference Kapperud4–Reference Ortiz Martínez7]. The occurrence of pathogenic Yersinia in raw pork may be up to 16·7% [Reference Nørrung and Buncic1]. In case-control studies, consumption of food prepared from raw pork products has been identified as a major risk factor for yersiniosis [Reference Tauxe8–Reference Boqvist11]. Similar genotypes have also been observed in Y. enterocolitica strains of human and pig origin, indicating that contaminated pork plays an important role in human yersiniosis [Reference Fredriksson-Ahomaa12, Reference Fredriksson-Ahomaa13].
Y. enterocolitica is rarely isolated from piglets. Young pigs start to shed the organism in their faeces at the age of 12–14 weeks [Reference Gürtler14, Reference Nesbakken15]. Shedding increases with age, reaching highest levels at 19–20 weeks after which shedding decreases gradually. At the age of slaughter, a high proportion of fattening pigs carry Y. enterocolitica in their tonsils [Reference Gürtler14, Reference Fredriksson-Ahomaa16, Reference Bucher17]. Highly contaminated tonsils can further contaminate carcases during the slaughter and evisceration process, particularly when the head is not removed and handled separately [Reference Fredriksson-Ahomaa16]. Y. enterocolitica has also been isolated from sows but the prevalence is significantly lower compared to young fattening pigs, possibly due to natural resistance [Reference Korte5]. However, sows may still be the source of infection in piggeries.
Variation in shedding and within-farm prevalence of Y. enterocolitica exists between farms [Reference Ortiz Martínez7, Reference Laukkanen18, Reference Ortiz Martinez19]. Factors associated with the within-farm prevalence in pig production have been investigated in several studies [Reference Laukkanen18, Reference Laukkanen20–Reference Wesley, Bhaduri and Bush23]. Farm location, vaccination against Escherichia coli, and deaths due to scours and meat or bone meal in the diet have previously been associated with high on-farm prevalence of Y. enterocolitica [Reference Wesley, Bhaduri and Bush23]. In addition, high production capacity, wet feeding, faecally contaminated feed, absence of coarse feed or bedding, and drinking from a nipple [Reference Laukkanen18], as well as presence of cats or kittens on a farm and use of straw bedding have been linked to high on-farm prevalence [Reference Skjerve20]. Attempts to isolate Y. enterocolitica from the farm environment in order to define a potential environmental source of infection have failed [Reference Gürtler14, Reference Pilon, Higgins and Quessy21] indicating that the environment plays a minor role in origin of Y. enterocolitica and pig-to-pig transmission is most important in the spread of infection. Pigs can acquire the infection from contaminated faeces or pen floors. Y. enterocolitica is also thought to spread within a piggery when pigs are regularly moved from one pen to another [Reference Fukushima24].
Some farms have consistently low levels of Y. enterocolitica [Reference Laukkanen18, Reference Bowman25]. In Norway, specific pathogen-free herds have successfully been established and maintained free from Y. enterocolitica for several years [Reference Nesbakken, Iversen and Lium26]. However, establishing new herds by caesarean section and completely renewing the swine population within a country is very expensive and laborious. A question remains: Could Y. enterocolitica be prevented on a pig farm by focusing on management and by determining the detailed factors related to the prevalence of Y. enterocolitica? Thus, the objectives of our study were to find factors associated with the within-farm prevalence of pathogenic Y. enterocolitica, to analyse which factors affect the risk of pigs being positive for Y. enterocolitica at the abattoir and to find risk factors for faecal shedding.
METHODS
Sampling and data collection
Intestinal contents and tonsils from 788 healthy fattening pigs from 120 different farms were sampled at six different slaughterhouses in Finland to investigate for the presence of Y. enterocolitica. The pigs were randomly chosen from the slaughter batch from each farm. On average, 13·1 samples were collected per farm (range 2–86, s.d.=15·68). Intestinal contents were collected with a sterile spoon through an incision in the bowel and tonsils were collected aseptically.
Pathogenic Y. enterocolitica was isolated and identified as previously described [Reference Laukkanen18]. Briefly, Y. enterocolitica was isolated using cold enrichment in peptone-mannitol broth (PMB) at 4°C for 7 and 14 days, followed by alkali treatment with 0·25% KOH solution. In addition, selective irgasan-ticarcillin-potassium chloride (ITC) enrichment at 25°C for 48 h was used. The samples were plated onto cefsulodin-irgasan-novobiosin (CIN) agar. The pathogenicity of the isolates was confirmed using PCR to detect the chromosomal ail [Reference Nakajima27] and the virF [Reference Kaneko, Ishizaki and Kokubo28] gene located in the virulence plasmid pYV. Strains were further tested using API 20E (bioMeriéux, France). The isolates were biotyped [Reference Wauters, Kandolo and Janssens29] and serotyped using commercial antisera (Denka Seiken, Japan).
The farms from where the sampled animals originated were first contacted by telephone to introduce the project and then mailed a purpose-designed questionnaire containing questions about production. In total, data on 166 variables were collected. The topics of the questionnaire are presented in Table 1. In addition, in order to clarify if the origin of piglets affects the carriage or shedding of Y. enterocolitica, the origins of examined fattening pigs were traced. These data were available from four out of six slaughterhouses comprising 73 of 120 farms (61%).
Table 1. List of the variables detected by the questionnaire in the present study
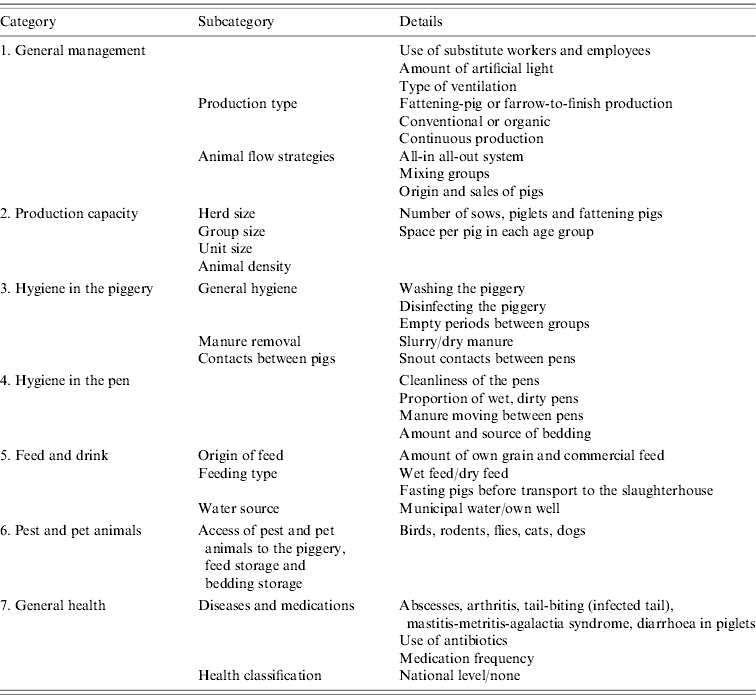
Background information
In Finland, pigs are generally fattened from about 25–100 kg. Piglets can be of various origins, and may come from a single farm or from several farms. Most fattening-pig farms operate an all-in all-out system, at least by unit. Most farrow-to-finish farms produce all the piglets by themselves while some purchase additional piglets. These farms typically have continuous production. Only those farms that supply fattening pigs to slaughterhouses were included in this study, with farms specializing in piglet production excluded. In Finland, only organic farms allow pigs access to outdoor areas.
The term ‘animal health classification’ relates to the prevention of contagious diseases by restricting the purchase and sales of animals, the origin of feed and connections to other domestic animals and pests.
Statistical analysis
A univariate statistical analysis with carriage and shedding as outcomes was conducted with random-effects logistic regression with farm as a clustering factor. Variables with a P value <0·15 were considered for inclusion into the respective multivariate random-effects logistic regression model where the outcome was carriage or faecal shedding of Y. enterocolitica in individual pigs. A pig being a carrier was determined by a pig having any positive sample. Both models were built by forward stepwise approach and a likelihood ratio test (LRT) with P⩽0·05 was used as an inclusion criterion. Each term was assessed for interaction. The reliability of the model estimates was assessed by comparing relative differences in the parameter estimates obtained by using different quadrature points, and by graphical exploration of deviance residuals and fitted probabilities. Cluster-specific odds ratios from each final model are presented. Stata 9 software (StataCorp LP, USA) and SPSS v. 15.0 (SPSS Inc., USA) were used for all statistical analyses.
RESULTS
The questionnaire was returned by 94 farms (78%). Of these, 55 were fattening-pig farms and 39 were farrow-to-finish farms. Some variation in the response rates in farms with different carriage prevalence was detected but the differences were not statistically significant. Of the 637 pigs included in this study, 318 (49·9%) were positive for pathogenic Y. enterocolitica. The tonsillar prevalence was 47·1% and faecal prevalence was 15·4%. Most of the pigs that were positive for Y. enterocolitica in faecal samples were also positive in tonsils. Eighteen pigs had positive faecal samples while their tonsillar samples were negative. Only seven (5·8%) of the farms in our study had all samples testing negative for pathogenic Y. enterocolitica.
The outputs of the univariate analysis for the factors associated with the carriage and shedding of Y. enterocolitica in this study are listed in Tables 2 and 3, respectively. Pigs from farms that practised organic production, used municipal water and purchased feed from a certain feed manufacturer, referred as company A, were less likely to be carriers of Y. enterocolitica (P<0·05) (Table 4). The protective effect of feed from company A was detected in farrow-to-finish farms rather than fattening-pig farms. Pigs from farms that used industrial by-products in feed, antibiotics every week and had a higher amount of artificial light in a piggery were more likely to be infected with Y. enterocolitica (P<0·05) (Table 4). The presence of pathogenic Y. enterocolitica in tonsils, buying feed from a particular feed manufacturer (company B), a higher level of animal health classification, the level of snout contacts between pigs and the use of tetracycline medication were found to have an association with increased faecal shedding (Table 5). Pigs from farms that used municipal water were less likely to shed Y. enterocolitica in faeces (P<0·01). Herd size, the distance of the farm to the abattoir and the proportion of own grain and commercial feed in diet were not associated with either the carriage or shedding prevalence of Y. enterocolitica.
Table 2. Results from the univariate analysis for carriage of Yersinia enterocolitica in pigs adjusted for farm clustering by random-effects logistic regression
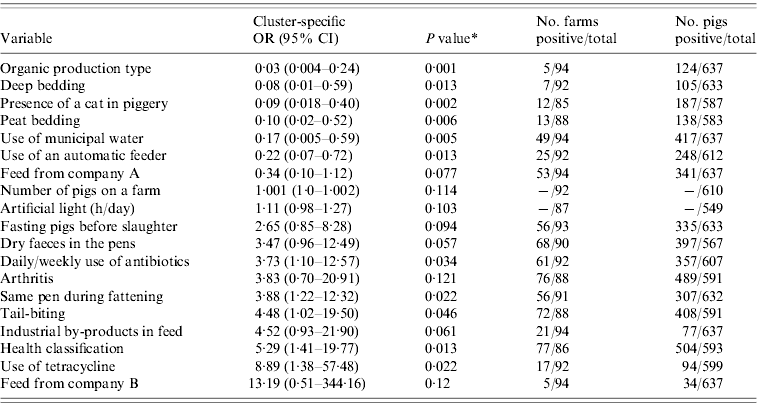
OR, Odds ratio; CI, confidence interval.
* P value from Wald test.
Table 3. Results from the univariate analysis for faecal shedding of Yersinia enterocolitica in pigs adjusted for farm clustering and tonsillar carriage by random-effects logistic regression
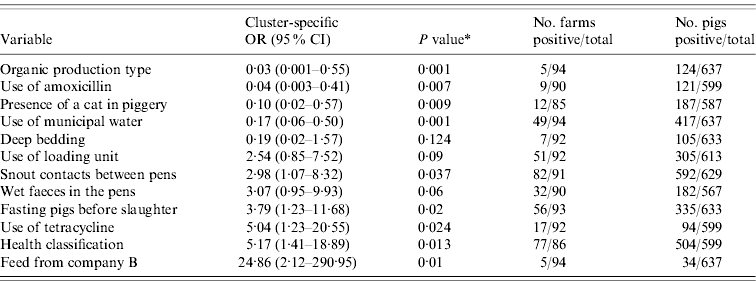
OR, Odds ratio; CI, confidence interval.
* P value from Wald test.
Table 4. Results from the final random-effects logistic regression model for a pig being a carrier (positive in any sample) of pathogenic Yersinia enterocolitica. Farm is the grouping factor. This model uses data from 85 farms and 519 pigs (average number of pigs per farm 6·1; min 1, max 36)
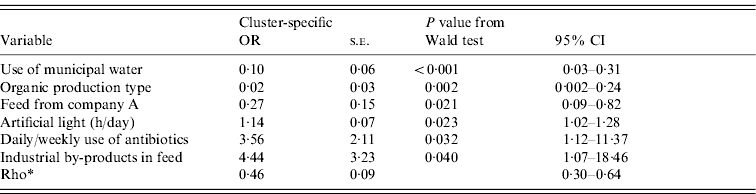
OR, Odds ratio; CI, confidence interval; s.e., standard error.
* Rho=within-farm correlation.
Table 5. Results from the final random-effects logistic regression model for a pig shedding pathogenic Yersinia enterocolitica in faeces. Farm is the grouping factor. This model uses data from 80 farms and 548 pigs (average number of pigs per farm 6·8; min 1, max 36)
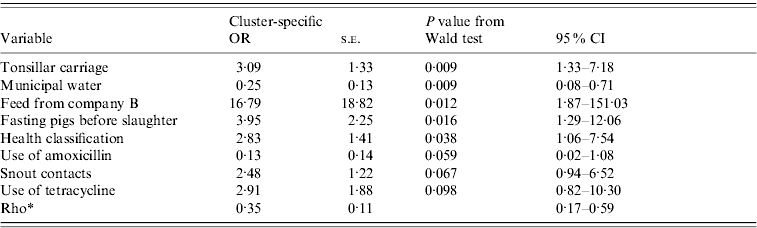
OR, Odds ratio; CI, confidence interval; s.e., standard error.
* Rho=within-farm correlation.
The production system (farrow-to-finish vs. fattening-pig system) was not significantly associated with carriage or shedding prevalence of Y. enterocolitica. The average number of farms supplying piglets to the fattening farm was 9·7 (range 1–45, s.d.=11·9) but had no effect on either total carriage or faecal shedding prevalence. Hygiene practices, including washing and disinfecting the piggery, shifting pigs from one pen to another and mixing groups were also not associated with the carriage or shedding of Y. enterocolitica.
Both multivariate models proved to provide reliable estimates, as the relative differences between the parameter estimates obtained by using different quadrature points remained very small. The deviance residuals were approximately normally distributed. The herd-level predicted probabilities with observed probabilities were in line with each other in both models (Fig. 1).
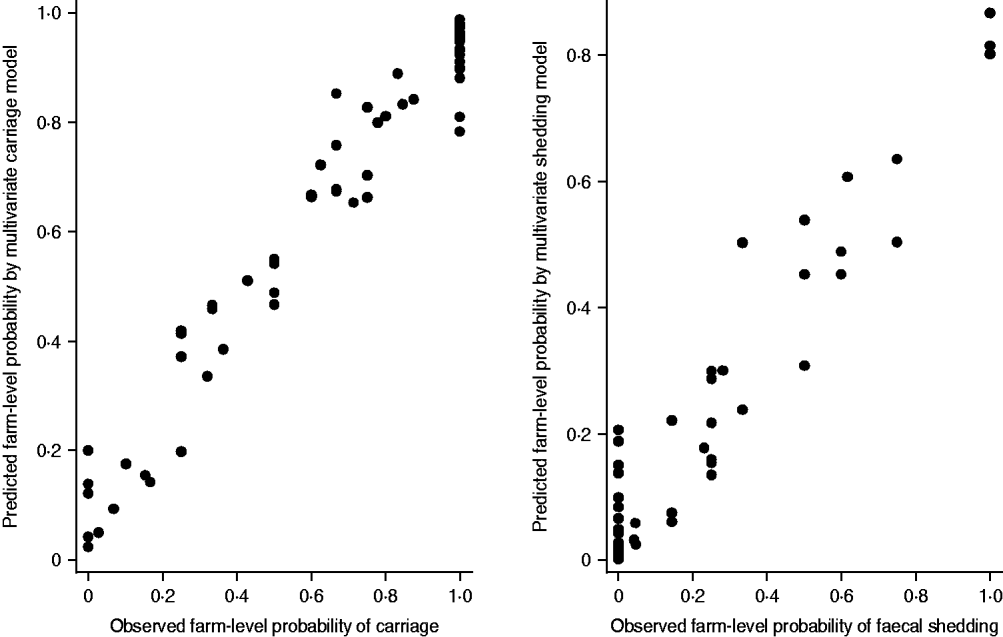
Fig. 1. The fit of the multivariate logistic regression models. The predicted probabilities of a pig being a carrier or a shedder of Yersinia enterocolitica on each farm are plotted against the observed farm prevalences. Only farms with three or more samples were included into the graph (n=70).
DISCUSSION
The sampling population is a representative sample of the Finnish fattening-pig population including pigs from both farrow-to-finish and fattening-pig production. However, since variation in response rates in farms with different within-farm prevalences existed, some caution must be taken when extrapolating the results to the wider population. We analysed the effect of a large number of variables compared to the sample size, although the variables chosen for this analysis were either previously suggested as risk factors, or known to affect the transmission probability of this pathogen. However, some variables may still be significant through chance alone and the stepwise method for inclusion in the multivariate model may exacerbate this problem. Due to the strong clustering by farm, the effective sample size required to detect associated factors remained fairly small, hence there may be additional factors that we did not find to be significantly associated due to a lack of study power.
Pathogenic Y. enterocolitica is widespread in the Finnish pig population. Only seven (5·8%) of the 120 farms in our study had samples that were all negative for pathogenic Y. enterocolitica. In the statistical analysis, we used a pig-level dichotomous positive or negative instead of farm-level positive or negative result, as has been performed in previous studies [Reference Skjerve20, Reference Wesley, Bhaduri and Bush23], taking the strong clustering by farm into account. We also analysed the carriage and faecal shedding of the pathogen separately in order to clarify the factors behind each of them. While the carriage of Y. enterocolitica can be related to the contamination of the carcases [Reference Fredriksson-Ahomaa16], we assumed that faecal shedding is likely to spread the infection within the piggery.
Tonsils are considered the most reliable tissue for identification of Y. enterocolitica from pigs [Reference Thibodeau30]. Besides our study, faecally positive samples alone have been observed in other studies [Reference Nowak22]. We assumed that faecal shedding without tonsillar carriage is a true finding. Furthermore, the statistical model produced in our study found factors other than tonsillar carriage that can explain faecal shedding. The sampling in our study was performed at the slaughterhouse; however, in order to avoid false results, samples should ideally be collected at the farm level to prevent pigs becoming contaminated during transport and at the slaughterhouse [Reference Funk31]. Since Y. enterocolitica infection originates from pig farms [Reference Laukkanen18], we concluded that the results obtained from the slaughterhouse mainly represent the situation on farms. Samples may still be contaminated at the slaughterhouse at the time of collection.
Several protective factors against pathogenic Y. enterocolitica were identified. Organic production type was one of the most significant protective factors and has previously been associated with low within-farm prevalence of Y. enterocolitica [Reference Laukkanen18, Reference Nowak22]. Based on our findings, low prevalence of Y. enterocolitica in organic farms is affected by generous use of bedding, limited use of antibiotics and lower animal density rather than the production type itself. Organic production is thus likely to cause less stress for the animals. Stress in animals can be a risk for food safety through various mechanisms [Reference Rostagno32]. Furthermore, organic pigs have lower daily weight gain compared to conventional pigs and are therefore slaughtered at an older age and thus may carry less Y. enterocolitica in their tonsils and intestines at the time of slaughter [Reference Gürtler14, Reference Nesbakken15]. In our study, the exact age of the fattening pigs at slaughter was unknown.
For both carriage and shedding of Y. enterocolitica, the use of municipal water was a significant protective factor. Y. enterocolitica is considered a possible waterborne pathogen [Reference Saebo33, Reference Sharma, Sachdeva and Virdi34] and has previously been isolated from tap water in a piggery [Reference Pilon, Higgins and Quessy21]. In total, 45 farms (48%) in the present study lacked access to municipal water and used instead untreated water from their own wells or from a small local water supply system. This is fairly common in sparsely populated areas in Finland. Water is likely to be a source of Y. enterocolitica infection for pigs.
Buying commercial feed from company A was found to be a protective factor against Y. enterocolitica while buying feed from company B was a clear risk factor. Commercial feed has previously been associated with herds that have high contamination of Y. enterocolitica [Reference Nowak22]. This is the first time that the protective effect of feed against Y. enterocolitica has been documented. The protective effect of feed from company A was particularly evident when the feed was used in farrow-to-finish production and thus fed to piglets. The ingredients of this feed were checked and were found to contain a prebiotic component designed to prevent the colonization of the intestine with other pathogenic bacteria belonging to the family Enterobacteriaceae. Thus, the prevention of pathogenic Y. enterocolitica seems to be possible with a dietary supplement. In the present study, the proportions of own grain and commercial feed had no association with the occurrence of Y. enterocolitica but the use of industrial by-products (whey or barley starch) was found to be a risk factor for Y. enterocolitica. These products are typically used with wet feeding. The use of industrial by-products in feed was also associated with larger herd size and higher daily weight gain of the pigs.
Hygienic barriers, built to prevent the spread of infectious diseases, seem to fail in prevention of Y. enterocolitica as previously observed [Reference Skjerve20]. In fact, higher animal health classification was a predisposing factor for faecal shedding in our study. In contrast to a previous finding [Reference Skjerve20], the presence of a cat on the farm was associated with the lower carriage prevalence. Washing and disinfecting the piggery was not associated with the carriage or shedding of Y. enterocolitica. Some farms reported disinfecting the piggery without first washing the organic material that is likely to inactivate the disinfectant and therefore the benefit of the procedure remains uncertain.
The prevalence of Y. enterocolitica has been significantly lower in production systems with limited number of piglet suppliers [Reference Skjerve20, Reference Nowak22]. In our study, the number of farms from which the piglets were purchased was associated with neither carriage nor shedding of Y. enterocolitica. In contrast to previous finding [Reference Fukushima24], no association was found between pig movement between pens and the carriage or shedding of Y. enterocolitica in the present study. Indeed, carriage prevalence was higher on those farms where pigs were held in the same pen during the whole fattening period. Instead, the hours per day with lights on, which we used as a proxy measure of pig activity, and the possibility for direct snout contacts between pens were associated with higher shedding prevalence. Activity time and contacts may influence the risk of direct disease transmission between pigs. Whereas the duration of light exposure affected the overall positivity, snout contacts were a risk factor for faecal shedding. The possibility for snout contacts between pens was classified by enquiring whether there were solid or open walls between pens that allowed pigs to have nose-to-nose contact with each other.
The daily and weekly use of antibiotics was related to higher carriage prevalence of Y. enterocolitica but was also associated with larger herd size and thus higher predisposition to diseases. According to the data in our study, the most widely used antibiotic in Finnish pig farms is penicillin. Y. enterocolitica produces β-lactamase enzyme and is therefore typically resistant to β-lactamase antibiotics, although variation exists between different serotypes [Reference Bottone3, Reference Bucher17]. Frequent use of penicillin may therefore provide an environment that allows rapid reproduction of the pathogen. Y. enterocolitica is susceptible to most other antibiotics including trimethoprim/sulfamethoxazole, amoxicillin/clavulanic acid, gentamicin and ciprofloxacin [Reference Bucher17, Reference Fredriksson-Ahomaa35]. Resistance against tetracycline has been documented in the USA [Reference Funk36, Reference Bhaduri37]. In Finland, 61% of the strains tested were susceptible to tetracycline (R. Laukkanen et al. unpublished observations) but no actual resistance has been detected. The use of tetracycline was related to higher carriage and shedding prevalence of Y. enterocolitica in our study. This finding may be a reflection of the overall lower health status of pigs on these farms, and therefore the need for tetracycline medication. The use of amoxicillin was a protective factor. The susceptibility of Y. enterocolitica to amoxicillin in Finland has not been studied to our knowledge.
This study offers a useful insight into the within-farm transmission patterns of Y. enterocolitica. Water may be an important source or means of transmission of the infection. Feed distributors and feed quality may also play a significant role. Veterinary antibiotic treatment practices were found to have an effect on the carriage and shedding of this zoonotic agent and hygienic barriers currently in place appear ineffective to deal with this pathogen. The present study has also demonstrated that differences in management practices can explain variation in the carriage and shedding of Y. enterocolitica on different pig farms. These findings can be used in further research to study the transmission dynamics of Y. enterocolitica.
ACKNOWLEDGEMENTS
This study was supported by research funding from the Ministry of Agriculture and Forestry, Finland (2849/502/2008) and was performed at the Centre of Excellence in Microbial Food Safety Research, Academy of Finland (118602). The farmers and the slaughterhouses are gratefully acknowledged for their cooperation. Special thanks to William De Glanville for revising the language.
DECLARATION OF INTEREST
None.








