Human pulmonary dirofilariosis is an emerging zoonotic disease caused by Dirofilaria immitis. Humans are accidentally infected in endemic areas, where dogs act as reservoirs of the parasite and the climate conditions favour the proliferation of the mosquito vectors [Reference Ferreira1]. D. immitis is considered a public health concern because of its zoonotic potential; in humans, the pre-adult parasite stages form coin-shaped cysts in the branches of the pulmonary arteries, which are generally asymptomatic. These cysts are detected by imaging techniques and are often mistaken for lung tumours. Biopsy is considered the definitive diagnostic method, although its invasive nature could be a limitation; on the other hand, in biopsy only late immature and adults removed before the degeneration process elicited by the inflammatory response of the host can be reliably identified, so polymerase chain reaction (PCR) could be a valuable tool in these cases. In turn, serology techniques, currently available only as ‘in house’ tests, could help to diagnose the nature of the pulmonary cysts, by detecting the presence of antibodies against D. immitis and its symbiotic bacteria Wolbachia; thus, human pulmonary dirofilariosis should be considered in the differential diagnosis of compatible lesions, especially in endemic areas [Reference Simón2, Reference Sileli, Tsagkaropoulos and Madesis3].
D. immitis causes heartworm disease in dogs and cats, which is endemic in Southern European countries, including Greece, Italy, Spain and Portugal. In these countries, the prevalence has increased in some regions [Reference Montoya-Alonso4, Reference Otranto and Dantas-Torres5], while others with consolidated prophylactic programs have reported decreased prevalence [Reference Montoya-Alonso6]. In humans, in 2012, there were reported 33 cases of pulmonary dirofilariosis in Europe, although, in endemic regions, the frequencies of human infections are probably higher than reported in the literature because pulmonary nodules may be unnoticed or be easily misdiagnosed [Reference Simón2].
Regions with high temperature and humidity favour mosquito proliferation and the presence of canine and human dirofilariosis [Reference Simón2]; moreover, the disease is expanding to colder areas in Eastern and Northern regions of Europe [Reference Morchon7–Reference Genchi9], as demonstrated by recent studies [Reference Simón2, Reference Svobodova and Misonova8–Reference Miterpakova16]. The limits of this expansion are fuzzy but could be higher than estimated given that cases have been diagnosed in dogs (although imported) from Nordic countries such as Finland [Reference Tiskina and Jokelainen15].
In Portugal, there is evidence of the presence of canine D. immitis infection in almost all regions of the country [Reference Vieira17–Reference Cardoso, Mendao and Madeira de Carvalho20]. Also, there is an increasing number of feline D. immitis infections in regions from Central, Northern and Southern Portugal [Reference Simón2, Reference Vieira18, Reference Maia21]. According to the Köppen-Geiger climate classification, Northern Portugal has a warm temperate climate with dry summers (type Cs), divided into two subtypes: Csa, with hot summers with the average temperature in the warmest month above 22 °C and Csb, with warm summers with the average temperature in the hottest month below or equal to 22 °C and with 4 months or more with the average temperatures above 10 °C [22].
To our knowledge, two previous reported cases of pulmonary nodules by D. immitis in Portugal demonstrated the risk of infection among the Portuguese population [Reference Araújo23]; however, no seroepidemiological study to assess this risk has been previously published. In the present study, we demonstrate, for the first time, the exposure to D. immitis of people living in Northern Portugal.
For this cross-sectional study, 668 human serum samples from two local hospitals (Centro Hospitalar S. João, Porto, Portugal and Centro Hospitalar de Trás-os-Montes e Alto Douro, Vila Real, Portugal) were analysed between July 2013 and November 2014. Inclusion criteria included people living in the area of interest of the study, who had not travelled outside the country in the last 6 months and agreed to participate. The samples were randomly selected among those who fulfilled the inclusion criteria.
Of the included samples, 333 (49.85%) were from males and 335 (50.15%) from females, ranging from 2 to 95 years (median 49 years, 36–67 interquartile range (IQR)). The number of samples by age group was 168 for ⩽35 years (25.10%), 193 for 36–50 years (28.90%), 133 for 51–65 years (19.90%) and 174 for ⩾66 years (26.0%). Serum samples were collected from people living in six districts of Northern Portugal, with Csa (Bragança, Vila Real) and Csb climates (Aveiro, Braga, Porto, Viseu). The pattern of distribution by age and gender was representative of the population living in Northern Portugal according to the 2011 census data [24]. The distribution of samples by gender, age and district of residence is shown in Table 1.
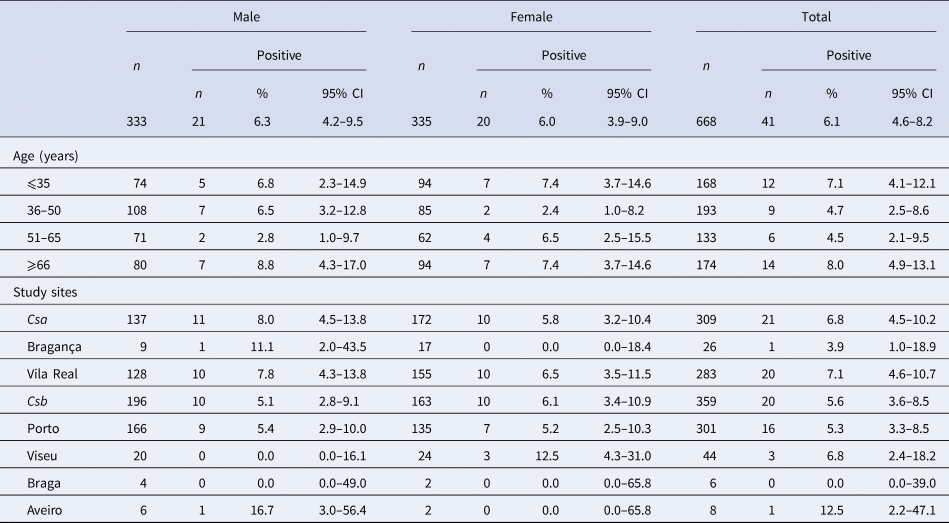
Table 1. Seroprevalence of human dirofilariasis in Northern Portugal, as defined by seropositivity for antibodies against both D. immitis and WSP
This research conforms to the Declaration of Helsinki and was approved by the ethics committee of the hospitals included in the study. The confidentiality of the information of the patients was always maintained and all of them provided written consent to participate in the study.
To estimate the seroprevalence of human D. immits exposure, samples were analysed by serological techniques for anti-D. immitis and anti-Wolbachia antibody detection, as previously described [Reference Simón25], with some modifications. In brief, 96-well microplates were coated with 0.8 µg of an extract of D. immitis somatic antigen and Wolbachia surface protein (WSP). Samples were prepared at 1:100 for anti-D. immitis serum antibodies and 1:40 for anti-WSP antibody detection. The secondary antibody (anti-human IgG peroxidase-conjugated; Merck, Germany) was used at 1:5000. An Easy Reader (Bio-Rad Laboratories, USA) was used to measure the optical densities at 492 nm. Cut-off points of D. immitis (0.8) and WSP (0.5) were obtained as the arithmetic mean optical density ± 3 standard deviations of 20 samples from clinically healthy blood donors living in a D. immitis-free area. People were considered seropositive when anti-D. immitis and anti-WSP antibodies presented jointly [Reference Montoya-Alonso4, Reference Vieira18, Reference Montoya-Alonso26].
Data were analysed using the SPSS® 25 software for Windows (SPSS Inc./IBM, Chicago, Illinois, USA). Descriptive analysis of the considered variables was carried out considering the proportions of the qualitative variables. The χ 2 or Fisher's exact tests were used to compare percentages of positives among categories of the same independent variables and also the total prevalence of D. immitis. The individual data were analysed as a dependent variable by the logistic regression model, using D. immitis status as the outcome. In all cases, the significance level was established at P < 0.05.
Of the studied samples, 41 were positive to anti-D. immitis and anti-WSP antibodies. Therefore, the seroprevalence was 6.1%. No statistically significant differences were observed between males and females (6.3% and 6.0%, respectively) and there were seropositive inhabitants from 19 to 88 years old (median age 50.0 years, 33.0–75.5 IQR). Seroprevalences were 7.1% (12/156) in inhabitants ⩽35 years, 4.7% (9/184) from 36 to 50 years, 4.5% (6/127) from 51 to 65 years and 8.0% (14/160) in those ⩾66 years (P > 0.05). Considering the climate areas, a prevalence of 6.8% in the Csa and 5.6% in the Csb climate zones was observed, with no significant differences between them (P > 0.05). The seroprevalences by district were 12.5% (Aveiro), 7.1% (Vila Real), 6.8% (Viseu), 5.3% (Porto), 3.9% (Bragança) and 0% (Braga). The baseline characteristics of the study population and its reactivity to D. immitis and WSP are summarised in Table 1. A map showing the study areas and the distribution of the positive subjects is shown in Figure 1.
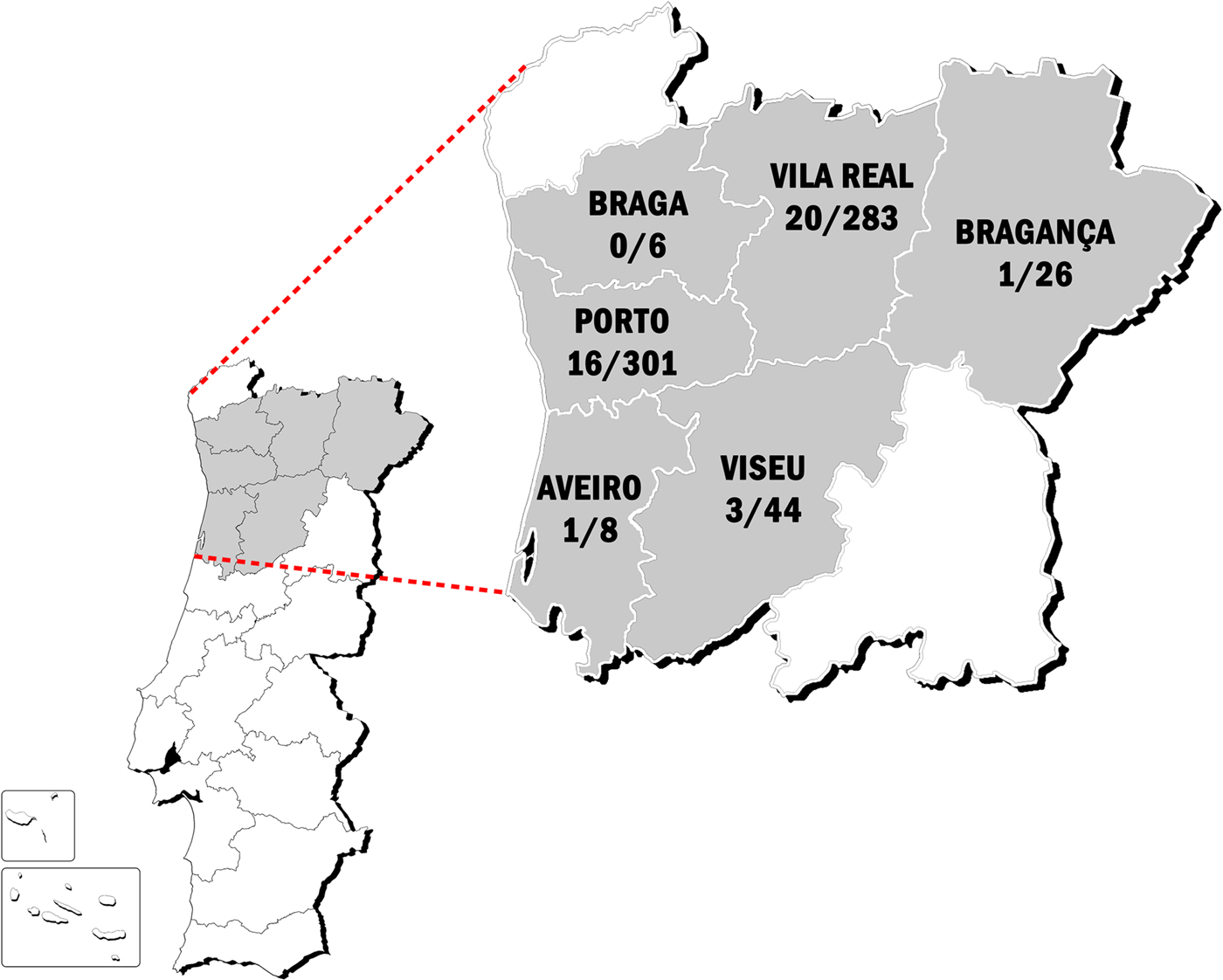
Fig. 1. Map of Portugal showing the areas included in the current study and the distribution of the positive subjects vs. the total subjects evaluated per area.
Finally, the univariate logistic regression analysis identified no statistically significant association between the evaluated independent variables (gender, age, climate area of residence) and positive serology to D. immitis antigens and WSP.
Heartworm infection prevalence is increasing in Western and Mediterranean European countries, while it is currently also being reported in Northern and Eastern areas of the continent. This is due to a number of factors, including climate change, the emergence of new species of mosquitoes able to transmit parasites, a greater transport of reservoir dogs from endemic areas and human modification of environment, such as irrigating lands for farming, which contributes to the spread of the dirofilariosis by allowing development and activity of the vector [Reference Morchon7].
D. immitis infection is a zoonosis and a vector-borne disease. Humans are exposed to this disease through the bite of infected mosquitoes and, as it is an emerging disease, it is not surprising that increasing of human dirofilariosis cases are being reported in endemic countries [Reference Simón2]. Zoonotic D. Immitis, as well as seropositive inhabitants, have been reported in Western Europe [Reference Simón25–Reference Foissac30] and Eastern Europe [Reference Ciuca13, Reference Kartashev31–Reference Arbune and Dobre34]. A similar pattern has been observed with D. repens, being more frequently reported in recent years [Reference Ciuca13, Reference Laynez-Roldan35, Reference Blaizot36].
Several studies confirmed Portugal as an endemic country for animal heartworm infection [Reference Alho37]. Regarding the area evaluated in the current work, a seropositivity of 15% in cats and between 2.1% and 27.3% in dogs has been observed [Reference Vieira18]. Similarly to as reported in other endemic areas, infected dogs seem to constitute a risk of transmission to human [Reference Montoya-Alonso38]. However, in areas where the seroprevalence of human heartworm infection was high (e.g. Porto, Vila Real and Viseu), no canine heartworm infection has been reported to date [Reference Vieira18]. Thus, further studies are needed to update the data of canine heartworm infection prevalence in these districts; however, being Portugal a small country, it facilitates travelling between endemic and non-endemic areas. On the other hand, the highest prevalence was found in Aveiro, where the presence of D. immitis has been demonstrated in dogs (6.8%) and cats (18.7%) [Reference Vieira18], suggesting that the inhabitants have a high risk of infection. Similar findings have been reported in the nearest country – Spain –in La Rioja [Reference Morchon27] and the Canary Islands [Reference Cabrera39]. There is a lack of seroepidemiological studies in other European regions, such as Eastern countries, although new studies are demonstrating the presence of seropositive inhabitants in Romania, Moldova and Serbia [Reference Ciuca13, Reference Tasic-Otasevic33]. Regarding transmission, presence of competent vectors as well as D. immitis infections has been described in mosquitoes in other areas of Portugal [Reference Santa-Ana, Khadem and Capela40, Reference Ferreira41] and neighbouring areas of Spain [Reference Bravo-Barriga42, Reference Morchon43].
The overall results presented the highest seroprevalence in inhabitants under the age of 35 years old (7.1%) and above the age of 66 years old (8.0%), similar to previous studies in other endemic areas [Reference Montoya-Alonso26, Reference Cabrera39].
Although pulmonary dirofilariosis has been described in Portugal [Reference Araújo23], awareness of human infections among Portuguese physicians is poor [Reference Belo, Afonso and Gonçalves44]. This reinforces the importance of the current study to raise awareness of the disease among the medical community. Importantly, physicians should be aware of this infection and should include pulmonary dirofilariasis in the differential diagnosis of patients presenting pulmonary nodules. Moreover, due to the increasing presence of canine heartworm infection, awareness campaigns should be carried out among veterinary clinicians and pet owners to promote the chemoprophylaxis of the disease [Reference Simón2, Reference Montoya-Alonso38].
Some limitations of this study should be considered, namely the limited number of samples obtained in some districts (e.g. Aveiro and Braga) and the evaluation of only a few independent variables (e.g. age, gender and area of residence). The influence of other variables on the outcome would have been important to consider (e.g. spending time outdoors during the mosquito season, travelling to endemic areas, owning dogs and presence or absence of D. immitis infection in them).
In conclusion, this study described, for the first time, the seroreactivity to D. immitis and WSP in the human population living in several districts of Northern Portugal. The presence of dogs infected by D. immitis is a potential threat to public health [Reference Ciuca13, Reference Simón25, Reference Montoya-Alonso26]. So, from the point of view of the One Health concept, it is necessary to build cooperation amongst physicians and veterinarians in the surveillance and control of this emerging zoonotic disease. Moreover, further studies are needed to understand the possible interactions between human and animal heartworm infection to guarantee its prevention and management.
Acknowledgements
The authors are grateful to the Serviço de Imuno-hemoterapia of Centro Hospitalar São João, Porto, Portugal, in particular, Dr Cristina Neves for her help during the study and to Centro Hospitalar de Trás-os-Montes e Alto Douro, Vila Real, Portugal, in particular Dr Fernando Caldeira for his help during the study. The authors are also grateful to Professor Luís Maltez da Costa for his precious help in drawing the map with the areas of study.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Centro Hospitalar de S. João (CES).




