Introduction
Diarrhoeal disease accounts for between 7% and 13% of deaths in children under-5 in Kenya [Reference Carvajal-Velez1]. In a 2015 Millennium Development Goal assessment conducted for Kenya, 15% of rural areas practiced open defaecation, and 36% had unimproved sanitation facilities [Reference Victoria2].
Latrine construction is a major component of sanitation improvement in developing countries. Community-led total sanitation (CLTS) is a latrine construction intervention programme with a goal of establishing behavioural change to achieve and sustain open defaecation-free (ODF) status in communities to reduce diarrhoeal illness [Reference Kar and Chambers3–Reference Mukherjee, Robiarto and Saputra6]. An ODF environment reduces diarrhoeal illness by enforcing proper faecal disposal methods, improving water quality and decreasing human-to-human pathogen transmission [Reference Patil7, Reference Mara8].
Risk factors and aetiology information for childhood diarrhoea and CLTS is limited in sub-Saharan Africa. Previous studies cited malnutrition, HIV status, breastfeeding, water fetching distance, poor hygiene or diet, younger age and caregiving as likely factors that increase the spread or severity of diarrhoeal disease [Reference Arvelo9–Reference Rao14]. Few studies have evaluated water quality differences between ODF and non-ODF communities in CLTS areas in sub-Saharan Africa. Determining risk factors for morbidity and mortality due to diarrhoeal disease is an important factor for evaluating CLTS’ effectiveness.
We designed an epidemiological study to examine the relationship between communities with 100% latrine usage (ODF) and non-usage communities (non-ODF) to estimate diarrhoeal disease prevalence in children ⩽5 years old in order to determine the success of the CLTS intervention programme, and how this may be influenced by water safety and sanitation and hygiene practices. Identifying risk factors for diarrhoea in children in latrine intervention communities allows for improvement in programme implementation and sustainability for the future.
Methods
Study area and population
This cross-sectional study was conducted in Ahero township, which is located in Nyando District near Lake Victoria in Kisumu County, Kenya [Reference Brooks15]. The district lies in the eastern part of the lowlands surrounding the Nyando Gulf and is prone to flooding, leading to epidemics of cholera and diarrhoea [Reference Brooks15, Reference Nyakundi, Mwanzo and Yitambe16]. The area is rural, with high poverty levels [Reference Brooks15, Reference Tiondi17]. Kisumu County has the nation's highest under-5 mortality rate (82 deaths per 1000 live births) and the second highest infant mortality rate (50 deaths per 1000 live births). The past 2 weeks under-5 diarrhoea prevalence was the second highest in the nation (18.9%). In Kenya, 66.3% of the population in rural areas reported using an unimproved toilet facility that does not conceal excreta from human contact, while 41.5% reported utilizing an unprotected drinking-water source that does not protect from contamination [18, 19].
The study was approved by the University of Illinois at Chicago Institutional Review Board and the Maseno University Ethics Review Committee. All participants provided verbal informed consent.
Data collection methods and instruments
The Ahero township's Ministry of Public Health and Sanitation (MOPHS) identified locations with designated ODF or non-ODF status. The ODF area had a total of three sub-locations and the non-ODF community had two sub-locations. We conducted simple random sampling stratified by sub-location for representativeness. We visited each household selected, and those with children age ⩽5 years with a parent or long-term caregiver present to respond to the questionnaire were eligible for entry. Households were excluded if there was no child ⩽5 years old present or if caregivers refused to participate. If a household member refused study entry or was ineligible, we continued to the nearest household [Reference Bennett20]. If there was more than one child ⩽5 years present and resident in the household, simple random selection using a random number generator function on a calculator was used to choose one child. Latrine use was not assessed for children too young ( < 5 years) to use toilets by themselves.
Using data from Kenya's Demographic Health Survey, we estimated a self-reported 16.2% prevalence of diarrhoea in children ⩽5 in the exposed (non-ODF) group [Reference Tiondi17]. We calculated a necessary enrolment of 396 subjects (198 children in each group) to detect a 9% absolute reduction in diarrhoea prevalence in the unexposed (ODF) group [Reference Patil7, Reference Garrett21–Reference Azurin and Alvero24]. This expected difference was a mid-point estimate based on the literature review of similar studies that found between no difference and a 64% reduction in diarrhoeal disease prevalence in children ⩽5 [Reference Garrett21–Reference Pickering28].
A standardised questionnaire collected: demographics factors, food handling, hygiene practices, latrine usage behaviours, diet, breastfeeding, co-morbidities, treatment, livestock proximity, diarrhoea in the past year and factors related to diarrhoea illnesses. The questionnaire was available in English, Kiswahili and Dholou. The survey was administered by MOPHS employees who were native speakers of the local languages.
Data collection method for laboratory analysis
Water samples were collected during interviews from study locations. Participants were asked where they obtain their drinking water and we sampled the corresponding sources. Sampling sources included public taps, boreholes, dug wells, springs, rainwater, ponds, rivers and streams [29]. Sampling procedures followed the Standard Operating Procedures for Determination of Total Coliforms and Escherichia coli in water using the 24 h Colilert methodology. Deviations from the procedure included the usage of Aquagenx Compartment Bag Tests (CBT) for the detection of E. coli [30]. An electronic turbidity meter was used to measure turbidity in nephelometric turbidity units (NTU, HF Scientific, model 2000). Per cent unsafe, median turbidity and per cent untreated were calculated for the sampled water sources within the five sub-locations (three ODF, two non-ODF) in order to apply the ecological-level results to the individual-level survey data. Water safety was dichotomised as safe (0 coliforms/100 ml) or unsafe (⩾1 coliform/100 ml) for analysis due to minimal variation of E. coli-level contamination across the WHO's original six health risk groupings among our collected samples. Sampled types of water sources were categorised according to the specific type, using unprotected and protected as the WHO identifies [29]. Sampled turbidity levels were grouped according to the WHO's drinking-water recommendation of ⩽5 NTU as ‘low’ and >5 NTU as ‘high’ levels.
Statistical analysis
The outcome for analysis was acute diarrhoea in the one past year, dichotomised as yes vs. no. Acute diarrhoea was defined as having three or more loose/watery stools within a 24 h period [Reference Arvelo9]. The primary explanatory variable was ODF status, examined as ‘ODF’ or ‘non-ODF’. Univariate analysis and descriptive statistics were computed. The frequency distribution of categorical variables by ODF status were compared using χ 2 tests. Modified Poisson regression with robust variance was used to approximate the log-binomial model to calculate prevalence ratios to measure the association between ODF status and having acute diarrhoea in the past year, with water safety, water source factors, latrine usage behaviours, demographic factors, hygiene and child's HIV status. Variables with a P-value < 0.10 were entered in a multivariable model. The latrine availability variable was removed from the model due to collinearity with the ODF variable. Backwards selection was conducted to determine which variables to retain in the model, using an α level of ⩽0.05 for statistical significance. All analyses were conducted using SAS Version 9.3 software.
Results
Study population
From 23 June 2015 to 7 July 2015, 426 households were surveyed via parents (87%) or caregivers (13%) of children ⩽5 years old. Of those surveyed, 210 were located in the ODF location and 216 were located in the non-ODF location. The response rate was nearly 99.8%. Latrines were located within 10 min walk of household clusters for 97% of ODF households and 76% of non-ODF households (P < 0.001). Overall, 82.6% were mothers to the child respondent, respondents were 13.9% HIV positive, 79.1% with primary school educational attainment, 79.0% reporting <2000 KSH per month household income and 39.5% employed as farmers. The population of respondent's children ⩽5 years was 51.6% female and 48.4% male, with an age range of <1 month to 5 years old (mean = 33 months; ±14.5 s.d.) and 4.9% HIV positive.
Factors associated with diarrhoea
Overall, survey respondents reported that 27.9% of children ⩽5 years old had diarrhoea for at least 3 days duration in the past year (Table 1). The occurrence of diarrhoea did not differ by location (ODF = 25% vs. non-ODF = 31%, P = 0.171). The mean age of children and distribution of gender did not differ by diarrhoea status (Table 1). In the context of water sources, a greater proportion of respondents reporting diarrhoea in their children collected water from natural sources (51.0%), compared with tap (13.7%) and wells (24.2%) (P < 0.001). The prevalence of diarrhoea was higher among HIV-positive (66.7%) than HIV-negative (27.3%) children (P < 0.001). The prevalence of reported diarrhoea was higher among children whose household had no income vs. higher income (56.3% vs. 27.6%, P < 0.001), and among those whose caretaker reported ‘no education’ compared with those with some education (44.4% vs. 26.2%, P = 0.010). Respondents without latrines were more likely to report diarrhoea in their children than those with latrines (56.9% vs. 23.4%, P < 0.001). The reported prevalence of diarrhoea was high for those disposing stools of on the ground (50%) and burying stools in the yard (47.1%), compared with 22% for respondents who reported throwing stools in the latrine (P < 0.001).
Table 1. Characteristics of subjects by reported diarrhoea status
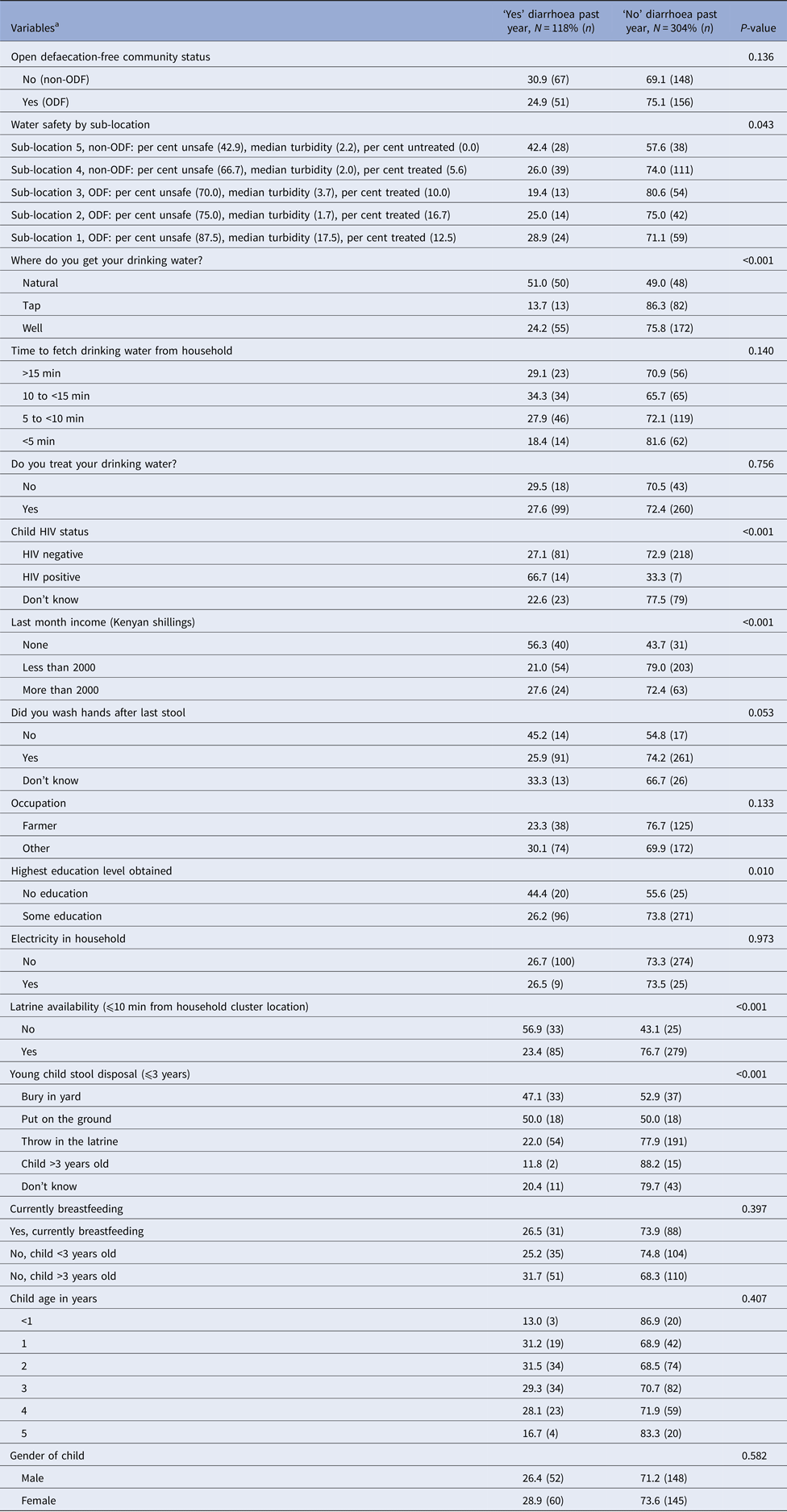
Note: Not all cells sum to N due to missing data; missing data ranged from 0.01% to 0.04%.
a Diarrhoea defined as three or more loose/watery stools within a 24 h period [Reference Guarino31].
Characteristics of non-ODF vs. ODF respondents
Compared with the ODF residents, respondents from the non-ODF community had lower monthly income, less education and less electricity (P < 0.05, each; Table 2). Respondents in the non-ODF location were more likely to be HIV positive compared with the ODF location (7.9% vs. 1.9%; P = 0.013). The majority of respondents fetched water from well sources for both locations, though this was higher among non-ODF than ODF participants (48.9% vs. 59.1%, P = 0.012; Table 4). Respondents from the non-ODF community more frequently reported no handwashing (10.2%) compared with the ODF community (4.3%; P = 0.041). A greater proportion of respondents in the non-ODF community reported burying children's stools in the yard (20.8%) and disposing children's stools on the ground (10.2%) compared with the ODF (yard = 11.9%; ground = 6.7%; P = 0.017). A higher proportion of respondents in the non-ODF location reported not having a latrine (23.6%) compared with the ODF location (3.3%; P < 0.001).
Table 2. Characteristics of subjects by location

Note: Not all cells sum to N due to missing data; missing data ranged from 0.01% to 0.04%.
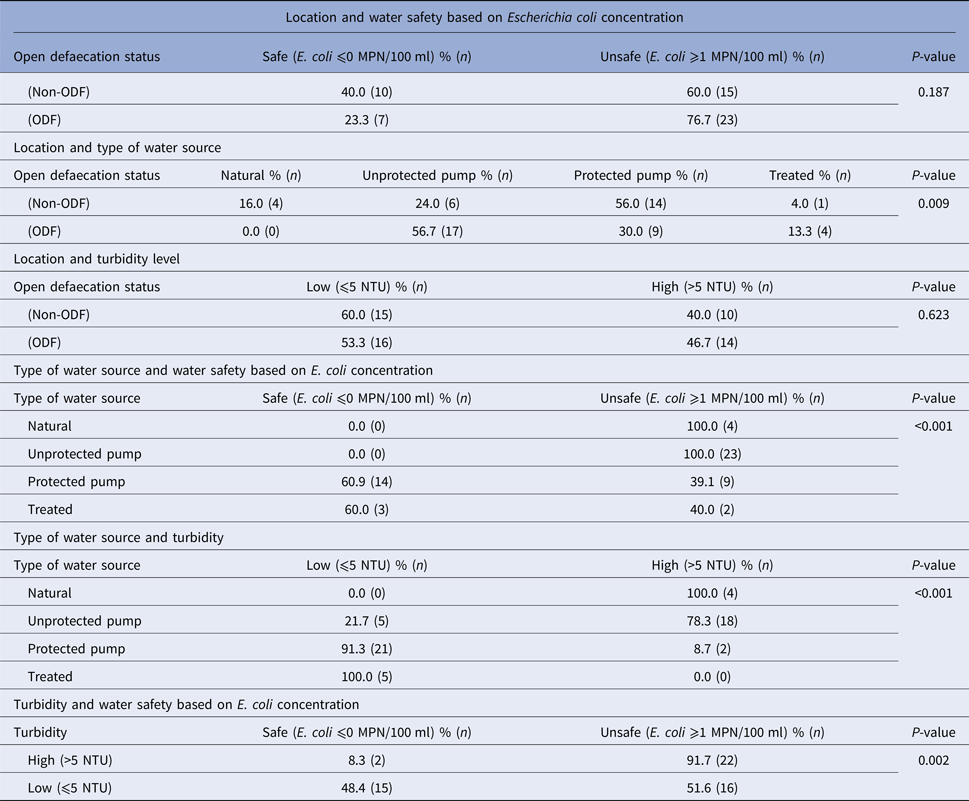
Water quality by location, safety, turbidity and water source type
Fifty-five water samples were collected from the ODF (N = 30) and non-ODF locations (N = 25). Overall, the ODF location had a higher proportion of unsafe water (76.7%) compared with the non-ODF location (60%), though this was not statistically significant (P = 0.187). Turbidity levels (high vs. low) did not differ by location (ODF = 58.3%, non-ODF = 41.7%, P = 0.623). However, the ODF community had a higher proportion of unprotected water pumps (56.7%) compared with the non-ODF (24.0%) community (P = 0.009). One hundred per cent of unprotected water sources were unsafe (>1 coliform/100 ml) compared with 39.1% of safe protected pumps (P < 0.001). Water samples from unprotected pumps had higher turbidity compared with protected pumps (high: 75.0% vs. 8.7%; P < 0.001). In general, higher turbidity levels correlated with unsafe water, while lower turbidity levels correlated with safe water (P = 0.002), which supports findings from E. coli measures (Table 3).
Table 3. Characteristics of water samples
Results of multivariable analysis: factors associated with diarrhoea
Covariates with P-value < 0.10 included: child's HIV status, hand washing after last stool, educational attainment, household cluster latrine availability, disposal of child's stool, household income and drinking-water source. In the crude analysis, children had a non-significant increased risk for diarrhoea if they lived in the non-ODF location compared with the ODF location (prevalence rate ratio (PRR) = 1.26; 95% CI0.87–1.95) (Table 4). After adjusting for child's HIV status, stool disposal and household income, the non-ODF community had a non-significant 16% higher risk of diarrhoea compared with the ODF community (adjusted PRR = 1.16, 95% CI 0.91–1.49). Additionally, HIV-positive children were more than twice as likely to have (adjusted PRR = 2.29, 95% CI 2.07–2.53) increased risk of diarrhoea compared with HIV-negative children. Respondents who reported burying children's stools in yards or putting stools on the ground had a 92% (95% CI 1.74–2.12) and 56% (95% CI 1.13–2.17) increased risk of diarrhoea, respectively, compared with subjects who throw children's stools in latrines. Households with no income had a 93% (95% CI 1.46–2.56) increased risk of diarrhoea compared with households with income of more than 2000 KSH per month (Table 4).
Table 4. Multivariable analysis of variables associated with diarrhoea status
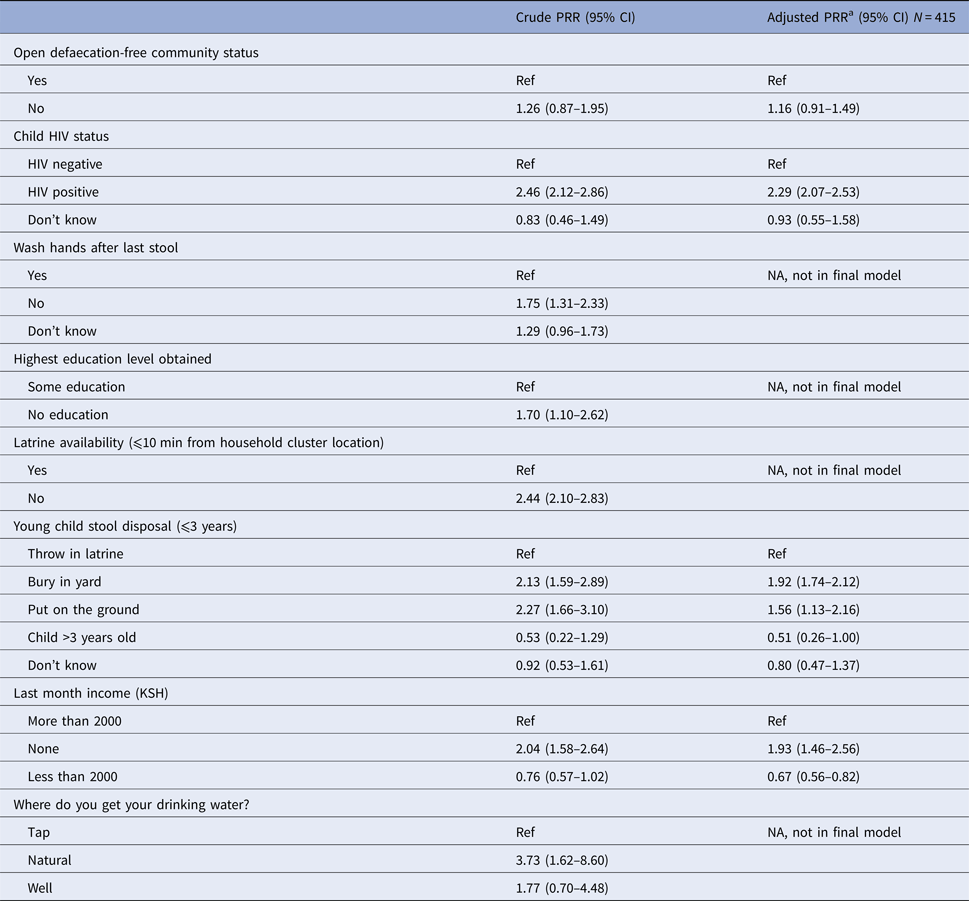
Ref, reference category; PRR, prevalence rate ratio; CI, confidence interval.
a Adjusting for ODF as a primary explanatory variable and all covariates presented.
Discussion
Summary of findings
Unexpectedly, there was no association between living in an ODF community and childhood diarrhoea status. Our findings suggest that water safety may have offset the relationship between ODF status and diarrhoeal status due to a higher likelihood of unsafe water consumption in the ODF community. Children's HIV positivity, parents safely disposing of young children's stools and low household income were positive risks for diarrhoea. In the context of modifiability, improved water treatment and safe stool disposal practices may reduce the risk of childhood diarrhoea in the non-ODF community. Safe water treatment practices may improve the CLTS intervention in ODF communities.
Explanation of results
Patil et al. conducted a cluster-randomised controlled trial of total sanitation campaign (TSC) to reduce open defaecation and various adverse health behaviours including water quality, compared with a group without TSC, in 80 rural Indian villages from 2009 to 2011. Similar to our study where we found no significant difference in child health after comparing intervention communities, there was no improvement in the rates of diarrhoea associated with intervention (7.4% intervention vs. 7.7% control). In contrast to our study's findings, reports of safe faeces disposal increased as a result of the intervention (27% intervention vs. 18% control; P-value < 0.001) [Reference Patil7]. Authors attribute intervention failure to variability in household latrine coverage (5–79% coverage), and variability in reported frequency of open defaecation in the intervention group (ranging from 32% to 97%) [Reference Patil7]. Also in contrast to our findings, a cluster-randomised controlled trial conducted in Nyanza Province, Kenya, in 2008 by Garrett et al. found that an improved latrine intervention led to a 69% reduction in caregiver reported diarrhoeal episodes over an 8-week period [Reference Garrett21]. Compared with our 92% increased risk of diarrhoea in respondents who disposed of stools in latrines to those who disposed of stools unsafely, a case–control study of environmental determinants and acute childhood diarrhoea in Ethiopia found an even higher (OR 3.35) odds of diarrhoea among children whose families disposed of infant faeces improperly [Reference Wanazahun and Bezatu32]. A more recent large cluster-randomised trial assessing the CLTS programme in Mali found no difference in diarrhoea prevalence between the intervention and control groups, although compared with our study, the latrine using community only had 65% latrine coverage. The authors did detect a six-percentage point reduction in stunting in children <2 years old, indicating an improvement in child health [Reference Pickering28]. In comparison to our twofold increased risk of diarrhoea in HIV-infected children, HIV-infected mothers were less likely to have improved latrine facilities compared with HIV-uninfected mothers from a study in Botswana that concluded an increased mortality rate for children <24 months old [Reference Zash33]. These studies demonstrate the variable impact of latrine interventions on childhood diarrhoeal health. Most studies assessing latrine intervention and child diarrhoeal health are caregiver-reported. Previous randomised trials have been unable to detect a difference in children's health between the CLTS/water sanitation hygiene intervention and non-intervention groups, citing confounding due to intervention communities not obtaining universal or 100% latrine uptake, latrines not adhering to sanitation standards, variation in E. coli contamination and human hygiene behaviours when comparing groups, potential increases in E. coli pathogen presence upon introduction of latrines in communities, and overall difficulties of obtaining community ODF status due to complications of latrine construction and maintenance practices [Reference Patil7, Reference Garrett21, Reference Pickering28, Reference Wanazahun and Bezatu32, Reference Zash33].
Recommendations
Based on the study's findings, public health officers should promote home-based water treatment interventions, in both communities. Officers should conduct education sessions to ensure community members are using proper methods to chlorinate or boil water. Introducing alternative methods for water treatment, solar disinfection, biosand or ceramic filters and plant treatment, may be another option to be sure that water is sanitised effectively [Reference Meierhofer and Wegelin34–38]. Public health officers should also emphasise safe disposal of children's faeces as a component of the intervention. Despite finding no difference in diarrhoeal rate by intervention location, latrine construction should still be supported in the non-ODF location considering the non-ODF location had a slight increase in risk compared with the ODF. Ideally, the combination of latrines, proper water treatment and safe stool disposal practices should reduce the risk of diarrhoea in the communities. Interventions may have more critical impact for children with HIV.
Strengths and weaknesses
We achieved the desired sample size of the study and were adequately powered to detect modest differences in diarrhoea by non-ODF vs. ODF location. Water quality testing acted as a biological measure and environmental indicator for contamination and helped explain why the diarrhoeal rates observed were similar by community. Water data were collected at the ecological level, which was appropriate given that the majority of households in Nyando constituency do not have private water source access.
Selection bias may have occurred in the way ODF and non-ODF locations were selected due to jurisdiction restriction of the MOPHS. These restrictions resulted in a higher prevalence of latrine coverage in the non-ODF group than anticipated (76% non-ODF vs. 97% ODF). Higher latrine coverage in the non-ODF community may have resulted in a smaller effect size when comparing the risk of diarrhoea disease in the non-ODF vs. the ODF. Our interviewing process occurred from 10am to 4pm during the week. This time frame may have caused us to miss children who were in school, which may have skewed our sample to children ⩽3 who are too young to go to school.
A past 4-day incidence was originally ascertained to avoid recall limitations. Following analysis, the overall past 4-day incidence was 8%, with no difference when comparing non-ODF vs. ODF communities (7.9% vs. 8.1%; P = 0.923). Therefore, the prevalence of diarrhoea in the past year variable was utilised to have optimal power for multivariable analysis.
Health behaviour survey questions related to hand washing, stool disposal, water treatment and latrine usage may be subject to providing socially desirable responses. However, we used direct observation from the interviewer (e.g., latrine presence), to strengthen the validity of data. Diarrhoea rates may have been underestimated due to the inability to collect valid latrine usage data in children <5.
Conclusion
Diarrhoeal disease rates in children ⩽5 in Nyando constituency of western Kenya did not differ by whether a latrine intervention was implemented. However, this may be due to more unsafe water and hygiene practices in the ODF location. We recommend water treatment interventions and educational interventions for improved sanitation practices to reduce the rates of diarrhoeal disease among children ⩽5. Without additional water treatment education and safe stool disposal practices, the CLTS initiatives may be ineffective.
Acknowledgements
We thank the families who participated in this study, and all of the staff of the Ministry of Public Health and Sanitation Ahero who contributed, especially Tom Omullo, John Mboya, Oduor Reagan, and Lameck Abok. We also thank Millicient Ogosi, Walter Agingu, and other Nyanza Reproductive Health Society staff for facilitating the Maseno University IRB submission, and providing a laboratory for water quality testing. The research in this publication was supported by the Douglas Passaro Global Horizons Award.
Author contributions
CB developed research question; study hypothesis and design; study protocol development; supervised field study team; conducted data collection; data analysis; interpretation of results; wrote first draft of the manuscript. SDM advised research questions, study hypothesis and design, protocol development, data collection methods, data analysis, interpretation of results, manuscript drafting, review, revision; general onsite study logistics. NM was involved in field/onsite supervision; advised data collection procedures; review and revision of the manuscript. IH advised design and development of laboratory methods and procedures; contributed to the interpretation of results; review and revision of the manuscript. PM assisted field/onsite supervision; assisted data collection procedures; review and revision of the manuscript. RB assisted with general onsite logistics; utilised laboratory facilities; assisted with onsite ethical review process; review and revision of the manuscript. RH consulted study protocol design; contributed to the interpretation of results; review and revision of the manuscript.







