INTRODUCTION
Ferritin is a ubiquitous and highly conserved iron-binding protein. In humans, iron in excess of need is stored primarily as ferritin, principally in the macrophage system of liver, spleen and bone marrow. Increasingly, perturbations in cellular iron and ferritin are emerging as an important element in the pathophysiology of disease. The changes in ferritin are important not only in the classic diseases of iron acquisition, transport, and storage, such as primary haemochromatosis, but also in diseases characterized by inflammation, infection, injury, and repair [Reference Torti and Torti1]. Among these are some of the most common diseases that affect human beings: Parkinson's and Alzheimer's disease, cardiac and neuronal ischaemia-reperfusion injury, atherosclerosis, pulmonary inflammatory states, rheumatoid arthritis, various pre-malignant conditions, and cancers [Reference Linert and Jameson2–Reference Wu8].
Not only as a member of a group of iron regulatory proteins that maintain cellular and organismal iron homeostasis, ferritin is also a member of the protein family that orchestrates the cellular defence against stress and inflammation. Described as a ‘two-faced’ protein, ferritin behaves as a positive acute phase reactant, whose serum levels rise rapidly in response to trauma, burn or inflammation [Reference Torti9]. The crucial role that ferritin plays in the cellular and organismal response to inflammation is intimately linked to its primary function in ‘iron sequestration’, which occurs in animals by sequestrating iron in a non-toxic form that minimizes its ability to catalyse the formation of reactive oxygen species (ROS). Therefore, iron status largely affects the generation of oxygen as well as ferryl and nitrogen radicals. In addition, the toxicity of iron at the cellular level is attributable in large part to its capacity to participate in the generation of such reactive species, which can directly damage DNA, lipids, and proteins, leading to profound cellular toxicity. Ferritin, by ‘capturing’ and ‘buffering’ the intracellular labile iron pool [Reference Picard10, Reference Picard11], thereby plays a key role in maintaining iron homeostasis and reducing the damage from pathogenic invasion.
Health disparities in different ethnic populations, especially between blacks and whites, has become a topic of intense research for the past few decades, yet they are still poorly understood. Of particular interest is the aetiology of ethnic differences in disease susceptibility and response [Reference Risch12]. Inflammation, the underlying pathophysiology of several disorders, is therefore suspected to be one of the factors that may contribute to the ethnic differences in disease progression and outcome. Immune system hyper-responsiveness and inflammation have been associated with a number of disorders that more commonly plague African Americans. Examples include preterm birth [Reference Ahern13, Reference Simhan14], atherosclerosis [Reference Wong15–Reference Losito17], autoimmune diseases [Reference Petri18], and transplant rejection [Reference Hoffmann19]. As a key molecule in cellular defence against stress and inflammation, ferritin limits the extent, character, and location of the pro-oxidant stress that typifies inflammatory diseases, cancer and conditions of altered oxygenation [Reference Pang4, Reference Ahmadzadeh7, Reference Biemond20]. Therefore, an understanding of how ferritin responds to inflammation may lead to insights into the aetiology of the difference in host inflammatory response and clinical outcomes.
In this study, we used data from the third National Health and Nutrition Examination Survey (NHANES III) to examine the ethnic difference in the relationship between acute inflammation (AI) and serum ferritin (SF). Our study thus had three purposes: (1) to examine whether the likelihood of having increased SF differs between subjects with and without AI; (2) to determine whether blacks are more likely to have elevated SF in regard to AI than whites, and (3) to investigate whether there are any differences in the magnitude of having elevated SF between blacks and whites with AI present.
METHODS
Data source
NHANES III was a national representative survey conducted by the National Center for Health Statistics between 1988 and 1994, to assess the health and nutritional status of the US population. The data were collected via standardized questionnaires, physical examinations and laboratory investigations. NHANES III included a sample of 33 994 persons aged ⩾2 months from 89 randomly selected locations throughout the United States. Multistage, stratified sampling design was applied to select the participants constituting a representative sample of the civilian, non-institutionalized national population. Additional details regarding the study design and sample selection were reported previously [21].
Study sample
A total number of 5007 NHANES III male subjects, aged ⩾20 years, with measured SF and C-reactive protein (CRP) was included in this study. Among them, 3112 were non-Hispanic white (NHW) and 1895 were non-Hispanic black (NHB). Due to the impact each has on ferritin, subjects who were haemophiliacs or who had recently undergone chemotherapy were excluded from the analysis. Subjects who had been treated for anaemia (including diet, iron pills, iron injections, transfusions as treatment) within the past 3 months or who had blood donation within 1 month prior to the survey were also excluded from the analysis. We only chose male subjects to eliminate the additional, potentially confounding effects associated with female subjects, such as menstruation, oral contraceptive use, pregnancy, parity, menopause and hormone replacement therapy.
Variables
Elevated SF was defined as a ferritin concentration >300 μg/l in men [Reference Barton22]. Presence of AI was defined as a CRP ⩾1·0 mg/dl [Reference Young, Gleeson and Cripps23]. Disease status including cancer (various types), congestive heart failure, diabetes mellitus, heart attack, hypertension, and stroke was determined by the responses to questions of whether a doctor has ever told the respondent that he had such conditions. Undiagnosed diabetes was identified by a fasting plasma glucose concentration >126 mg/dl [24]. Hypertension was defined as a systolic blood pressure ⩾140 mmHg or/and diastolic blood pressure ⩾90 mmHg [Reference Chobanian, Bakris and Black25], or if a doctor has told the participant that he had hypertension.
Several inflammation markers including plasma fibrinogen, serum albumin, white blood cell count (WBC), lymphocyte number, monocyte number and platelet count were examined. Other laboratory variables including haemoglobin (Hb), serum iron, transferrin saturation (TS), total iron binding capacity (TIBC), total triglycerides (TG), total cholesterol, high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol were also included in our study. Detailed description of laboratory measurements of these variables has been published elsewhere [Reference Gunter, Lewis and Koncikowski26].
Ethnicity, age, education, poverty income ratio (PIR), body mass index (BMI, kg/m2), and cigarette smoking may be associated with both AI and the levels of SF. These variables were, therefore, included in the logistic regression models as potential confounders. Age was modelled as a continuous variable, and was further divided into seven groups: 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ⩾80 years, in order to present the age-stratified distribution of elevated SF between black and white subjects with AI present. Education was measured as the highest year or grade completed by the respondent, and was further dichotomized as higher than or less than high-school education. PIR was measured based on total household income adjusted for household size, and was used as an index of relative socioeconomic status in the NHANES III survey. Three PIR strata: <1·3, 1·3–3·5, and >3·5 were used to represent low, moderate and high income levels, respectively according to the analytical and reporting guidelines of NHANES III [27]. BMI was further divided into four categories: underweight (BMI<18·5), normal (BMI ⩾18·5 to <25), overweight (BMI ⩾25 to <30), and obese (BMI⩾30) [28]. Cigarette smoking was classified as never smoked (if they had smoked <100 cigarettes in their lifetime), former smoker (⩾100 lifetime cigarettes, not currently smoking), and current smoker (⩾100 lifetime cigarettes, currently smoking).
Statistical analysis
The aims of our analyses were, first, to examine whether the likelihood of having increased SF differ between subjects with and without AI, within each ethnic group. This was determined by using multivariate logistic regression models to compare the odds ratios of having increased SF between subjects with and without AI present, stratified by ethnicity, after adjusting for the potential confounding effects.
The second aim of our study was to investigate whether blacks are more likely to have elevated SF than whites in regard to AI. This was determined by comparing the odds of having elevated SF between blacks and whites, stratified by AI status, after adjusting for the potential confounders.
The third aim of our analyses was to examine whether the magnitude of the elevated SF is different between blacks and whites with AI present. This was determined by comparing the risks of having increased SF per unit change in several inflammation markers including CRP, fibrinogen, WBC, albumin and platelet count between black and white subjects with AI present, after adjusting for the potential confounding effects. An interaction term constructed as cross-product of ethnicity and selected inflammation markers (as continuous variables) was included in the logistic regression models to allow calculation of β coefficients.
In addition to the confounders described earlier, cardiovascular diseases/diabetes mellitus/hypertension status, and cardiovascular disease risks including TG, total cholesterol, HDL cholesterol, LDL cholesterol, waist circumference, systolic and diastolic blood pressure were also adjusted for in logistic regression models because of their known relationship to ferritin and CRP [Reference Mainous29]. However, LDL cholesterol and waist circumference were excluded from logistic models because of their unacceptable percentage of missing values (LDL cholesterol 59·65%, waist circumference 15·54%). Multicollinearity among independent variables for each logistic model was tested by checking the variance inflation factor (VIF). Any variable with a VIF that exceeded 4 was excluded from the model (no variable was detected with VIF greater than 4).
Data were analysed by using SAS 9.1 (SAS Institute Inc., Cary, NC, USA) and SUDAAN 9.0 (Research Triangle Institute, Research Triangle Park, NC). Descriptive statistics were computed for all variables, including means for continuous data, frequencies for categorical variables, and standard error of the mean (s.e.m.). SUDAAN's t test and SUDAAN's χ2 test were used to compare the means of all continuous variables and frequencies of all categorical variables between NHWs and NHBs stratified by status of AI, respectively. Logistic regression analysis was applied for our likelihood and magnitude tests. The statistical significance level was set at P<0·05. Sampling weights were applied to all analyses to account for the complex design effect and for non-response.
RESULTS
Subjects characteristics
The overall prevalence of AI (CRP ⩾1·0 mg/dl) was 7·97% (399/5007), including 235 NHWs and 164 NHBs (Table 1). The mean SF was significantly higher in NHBs than NHWs (212·2 μg/l vs. 175·9 μg/l, P<0·01). In individuals with AI present, NHBs were younger, poorer, less likely to be a former smoker but more likely to smoke currently, and had a higher percentage of subjects with less than high school education compared to NHWs. Overall, there was no significant difference in reported disease prevalence including cancer, congestive heart failure, diabetes mellitus, heart attack, hypertension and stroke between NHW and NHB subjects with AI. There were no significant differences in the values of CRP, serum albumin, platelet count, plasma fibrinogen and lymphocyte between NHBs and NHWs. However, NHWs with AI present were found to have significantly higher values of WBC and mononuclear counts compared to their black counterparts. NHBs had significant lower values of Hb, but their SF concentrations were significantly higher compared to NHWs. Significant differences in HDL and LDL cholesterol were also found between these two groups (Table 1).
Table 1. Characteristics of 5007 male NHANES III participants aged ⩾20 years by status of acute inflammation (AI) and ethnicity

CRP, C-reactive protein; WBC, white blood cell; TS, transferrin saturation; TIBC, total iron binding capacity; HDL, high density lipoprotein; LDL, low density lipoprotein.
* The means of all continuous variables were compared between AI present non-Hispanic white and non-Hispanic black male subjects using SUDAAN's t test. The frequencies of all categorical variables were compared between these two groups by using SUDAAN's χ2 test. Statistical significance was set at P<0·05.
** The means of all continuous variables were compared between AI absent non-Hispanic white and non-Hispanic black male subjects using SUDAAN's t test. The frequencies of all categorical variables were compared between these two groups by using SUDAAN's χ2 test. Statistical significance was set at P<0·05.
For subjects without AI, significant differences in age, education, income level and smoking status were found between NHWs and NHBs. Generally, NHBs were younger, less educated, more likely to be non-smokers and current smokers, and had less income compared to their NHW counterparts. Disease status was not significantly different between the two groups, except for the significantly higher prevalence of heart attack in NHWs. The overall BMI and its categories were not found to be significantly different between NHBs and NHWs, but NHWs were observed to have significantly higher waist circumference compared to NHBs. All the infection markers were found to be significantly different between these two groups except for plasma fibrinogen. Haematological markers were also significantly different between NHWs and NHBs. Compared to NHWs, NHBs had significantly lower Hb but higher SF concentration. NHWs showed significantly higher levels of TG, total cholesterol and significantly lower levels of HDL cholesterol compared to NHBs. However, systolic blood pressure was significantly higher in NHBs (Table 1).
Distribution of elevated SF
The proportion of subjects with AI present who had elevated SF concentrations (>300 μg/l) stratified by age and ethnicity is shown in the Figure. NHBs generally showed a higher proportion of individuals with elevations in SF in regard to AI than their white counterparts at each age group except for age 20–29 years. The greatest ethnic difference in the percentage of individuals with elevated SF concentrations was seen in the 40–49 years age group.

Fig. The proportion of subjects with acute inflammation present who had elevated serum ferritin concentration (>300 μg/l), stratified by age and ethnicity. ■, Non-Hispanic black; □, non-Hispanic white.
The prevalence of elevated SF was significantly higher in NHBs (35·23%) with AI present than NHWs (21·78%). In the absence of AI, the prevalence of elevated SF was also significantly higher in NHBs (18·71%) than NHWs (13·14%). Generally, the proportion of subjects with elevated SF was lower in subjects without AI than in subjects with AI. Regardless of AI status, the proportion of individuals with normal ferritin concentrations was significantly higher in NHWs compared to NHBs (Table 2).
Table 2. Association between serum ferritin levelsFootnote † and acute inflammation (AI) status, by ethnicity

† A serum ferritin concentration <25 μg/l, 25–300 μg/l, or >300 μg/l was used to define decreased, normal and elevated ferritin level, respectively. SUDAAN's χ2 test was used to compare the number of individuals with decreased, normal, and elevated ferritin level between blacks and whites, respectively, stratified by AI status.
* Significant difference between AI present non-Hispanic white and non-Hispanic black male subjects, P<0·05.
** Significant difference between AI absent non-Hispanic white and non-Hispanic black male subjects, P<0·05.
Multivariate analysis
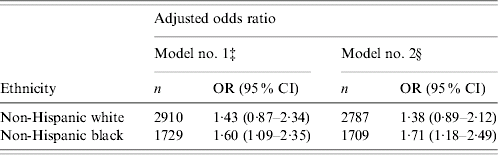
Compared with NHB males without AI, NHBs with AI present were 1·60 times more likely (95% CI, 1·11–2·35) to have elevations in SF after adjustment for age, PIR, education, BMI, smoking status, and disease status. When the relationship was further adjusted for cardiovascular disease risks including TG, total cholesterol, HDL cholesterol, systolic and diastolic blood pressure, NHBs with AI present were associated with a 1·71-fold greater risk (95% CI 1·18–2·49) of having elevated SF than NHBs without AI. In contrast, there was no significant association between elevated SF and AI found in NHWs (Table 3).
Table 3. Association between acute inflammation (AI) and elevated serum ferritin (SF) concentration, stratified by ethnicityFootnote †
OR, Odds ratio; CI, confidence interval.
† Odds ratios of having elevated SF concentrations were compared between AI present (CRP ⩾1·0 mg/dl) subjects and AI absent subjects (reference group) within each ethnicity. Elevated SF concentration defined as >300 μg/l for male.
‡ Model no. 1 adjusted for age, poverty income ratio, education, body mass index, smoking status, chronic diseases including cancer, congestive heart failure, heart attack, hypertension, diabetes and stroke.
§ Model no. 2 further adjusted for cardiovascular risk factors including triglycerides, total cholesterol, high density lipoprotein cholesterol, systolic and diastolic blood pressure in addition to the effects adjusted in model no. 1.
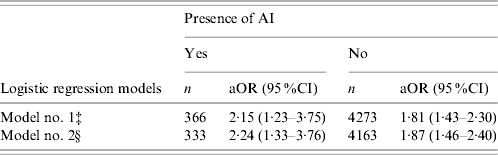
Irrespective of AI status, NHBs were more likely to have elevations in SF than NHWs (Table 4). With AI present, NHBs were 2·15 times more likely (95% CI 1·23–3·75) to have elevations in SF compared to their white counterparts after adjusting for age, ethnicity, PIR, education, BMI, cigarette smoking and disease status. The relationship remained significant after further adjustment for cardiovascular risks, and the odds ratio of having elevated SF in NHBs was 2·24 (95% CI 1·33–3·76). In the absence of AI, NHBs had a 1·81-fold greater chance (95% CI 1·43–2·30) of having elevated SF than did NHWs. After additional adjustment for cardiovascular risks, the odds ratio of having increased SF in NHBs was 1·87 (95% CI 1·46–2·40) (Table 4).
Table 4. Comparisons of non-Hispanic whites and non-Hispanic blacks in regard to the risk of having elevated serum ferritin (SF), stratified by acute inflammation (AI) statusFootnote †
aOR, Adjusted odds ratio.
† Odds ratios of having elevated SF in regard to AI were compared between non-Hispanic white (reference group) and non-Hispanic black male subjects, stratified by AI status. Elevated SF defined as >300 μg/l for male.
‡ Model no. 1 adjusted for age, ethnicity, poverty income ratio, education, body mass index, smoking status, chronic diseases including cancer, congestive heart failure, heart attack, hypertension, diabetes and stroke.
§ Model no. 2 further adjusted for cardiovascular risk factors including triglycerides, total cholesterol, high density lipoprotein cholesterol, systolic and diastolic blood pressure in addition to the effects adjusted in model no. 1.
With AI present, every increment of WBC, serum albumin, lymphocyte count and platelet count was associated significantly higher risks of having elevated SF in NHBs than NHWs in both reduced and full logistic models (Table 5). With additional adjustment for cardiovascular disease risks in the full logistic regression model, every unit change in CRP was associated with significantly higher odds ratios of having elevations in SF in NHBs than NHWs. However, no significant difference in the odds ratios of having elevated SF with unit change in monocyte count was found between NHBs and NHWs (Table 5).
Table 5. Comparisons of having elevated serum ferritin per unit change in inflammation markers between non-Hispanic whites and non-Hispanic blacks, with acute inflammation presentFootnote †

† Logistic regression models were used to estimate the magnitude of serum ferritin elevation associated with one unit change in inflammation markers. Interaction term (composite of ethnicity and selected infection markers as continuous variables) was included to allow calculation of β coefficients.
‡ Model no. 1 adjusted for age, ethnicity, poverty income ratio, education, body mass index, smoking status, chronic diseases including cancer, congestive heart failure, heart attack, hypertension, diabetes and stroke.
§ Model no. 2 further adjusted for cardiovascular risk factors including triglycerides, total cholesterol, high density lipoprotein cholesterol, systolic and diastolic blood pressure in addition to the effects mentioned in Model no. 1.
¶ P value corresponding to the comparisons between the β of non-Hispanic black and non-Hispanic white (reference group), respectively.
* Statistical significance set as P<0·05.
DISCUSSION
In the present study, the prevalence of elevated SF was significantly higher in NHBs than NHWs, regardless of AI status. Overall, the mean SF concentration of NHBs was significantly higher than that of NHWs. This finding is consistent with the results from previous studies in which blacks were found to have higher SF concentrations than their white counterparts [Reference Williams30–Reference Perry33]. Although most probably the higher SF in blacks results from a complex interplay of individual attributes, minority, socioeconomic stress and environmental characteristics, the possible influence of different response to inflammation on the ethnic differences in ferritin distribution has caught increasing research interest.
In the present study, NHBs consistently had a higher percentage of individuals with elevated SF than that of NHWs after age 30 years, when AI was present. In contrast to NHWs, NHBs were more likely to have elevated SF with respect to AI. Moreover, with AI present, NHB ethnicity was not only associated with greater risks of having increased SF, but also associated with a much greater magnitude of elevations in SF per unit change in inflammation markers. Therefore, the results raised the possibility that there might be different modes of inflammation-mediated ferritin increase between blacks and whites.
Cytokines directly regulate ferritin secretion both transcriptionally and post-transcriptionally. A number of cytokines including interleukin-1-alpha (IL-1α), interleukin 6 (IL-6), tumour necrosis factor-alpha (TNF-α) have been shown to up-regulate ferritin production [Reference Torti and Torti1, Reference Torti9]. Pro-inflammatory cytokines TNF-α and IL-1α transcriptionally induce the H chain of ferritin. As a result, the selective induction of ferritin H mRNA will lead to the accumulation of a population of H-rich ferritin protein and substantially increase the content of ferritin [Reference Torti and Torti1]. In primary cultured human hepatocytes, IL-1α and IL-6 can induce a transient secretion of ferritin at 24 h followed by a decline to baseline [Reference Muntanerelat34]. Results from these studies suggest another possibility that the differential patterns of raised ferritin observed between blacks and whites could be a result of different cytokine-induced ferritin synthesis during inflammation. However, prospective studies are required to test the hypothesis.
In our study, NHBs demonstrated a greater capability and propensity to have increased stored iron, reflected as elevated SF, than NHWs in regard to AI. One potential explanation for this finding is that the observed disparate patterns of ferritin increase may be a result of the different cytokine genotype distribution between blacks and whites. Several studies have shown that significantly different allelic and genotypic cytokine distribution exists between blacks and whites [Reference Cox35–Reference Ness37]. Most studies reported that blacks have a greater predisposition to inflammation because of the more up-regulated inflammation cytokine genotypes they carry, IL-1α, IL-6 and TNF-α in particular. Therefore, the higher risks of having raised SF observed in blacks with inflammation present may be the functional consequences of their predisposition to the higher levels of pro-inflammatory cytokines which are known to stimulate ferritin secretion.
We also observed that, with AI present, every unit increment of selected inflammation markers was associated with significantly higher risks of having elevated SF in NHBs than in NHWs. The stronger inflammation–ferritin relationship observed in blacks raised another possibility that the amplified increase in stored iron may predispose blacks to worse clinical outcomes and poorer treatment responses. Indeed, observations over the last few decades have identified ethnic differences in response to therapies in many diseases, including hypertension [Reference Seedat38, Reference Weir39], diabetes [Reference Lillie-Blanton40], renal transplantation [Reference Neylan41], and heart failure [Reference Carson42]. In addition, there are striking ethnic differences in response to interferon therapy for hepatitis C (HCV). Long-term sustained responses to alpha interferon are substantially lower in black patients than white patients with HCV [Reference Reddy43]. Interestingly, the fact that iron depletion by phlebotomy in patients with HCV reduces serum aminotransferase levels [Reference Di Bisceglie44], and in combination with interferon, may have improved antiviral efficacy compared with interferon alone [Reference Fontana45] suggests that increased iron stores may be the cause of the reduced response to interferon. Although the presence of increased iron stores is a recognized predictor of poor response to interferon monotherapy [Reference Clemente46–Reference Kageyama48], the association between increased iron stores and treatment response for other diseases has not yet been adequately investigated. Given the fact that ferritin plays a prominent role in the cytokine response and inflammatory processes, perhaps the more aggressive iron accumulation pattern, reflected by the higher likelihood and greater magnitude of elevated SF is a reflection of the greater intensity of inflammation and the stronger host immunoresponsiveness underlying a number of physiopathological processes. Therefore, larger, prospective studies are needed to investigate whether the different patterns of increase in iron stores are associated with different therapy response among various ethnicities.
To date, little is known about the underlying mechanisms of the disease disparities highlighted by the strikingly higher morbidity and mortality rates in blacks. Compared with whites, blacks have ~1·5 times higher death rates from all causes, worse survival after myocardial infarction [Reference Gillum, Mussolino and Madans49], a twofold-higher incidence of and morbidity from stroke [Reference Sacco50, Reference Broderick51], and significantly higher morbidity and mortality rates from diabetes [Reference Carter, Pugh and Monterrosa52], AIDS [Reference Murrain53], and cancer [Reference Parker54]. Given the pivotal role ferritin plays in the cellular inflammatory response and pathogenesis of disease, the question of whether there is a difference in responsive ferritin synthesis during inflammation between blacks and whites, and whether the differences in iron store play a role in disease disparities merits further research.
To our knowledge, this is the first study to assess the ethnic difference in the association between AI and increased iron stores, defined as elevated SF between blacks and whites. Despite the fact that ethnic differences in disease burden, progressions, complications, outcomes and treatment response have been observed between blacks and whites, the explanation for this remains unknown. Of great interest is to discover whether there are ethnic differences in the inflammatory process and host immunoresponsiveness that underlie a variety of clinical pathophysiological conditions. Within this work, we presented evidence that blacks seem to have a more aggressive pattern of having elevated SF in regard to AI compared to whites. And the stronger inflammation–ferritin relationship observed in blacks may reflect their more robust immune response and, therefore, may contribute to the poorer prognosis and treatment resistance. However, more prospective data are required to further investigate the hypothesis.
Despite the significant findings provided, our analysis is not powered to address the underlying mechanisms of the observed ethnic differences in the association between AI and raised ferritin. Due to the cross-sectional nature of the design, causal relationship cannot be inferred between AI and raised SF. Therefore, more specific investigation is needed to further explore the responsible biological rationale. However, our study does raise the possibility that different patterns of iron accumulation may play a role in disease processes and result in different clinical outcomes and drug response. Future studies will address this topic.
In conclusion, the current results indicated that different patterns of iron accumulation in regard to AI exist between blacks and whites. Further elucidation is needed about how ferritin regulation is perturbed in diseases, and whether increased iron stores play a role in different clinical outcomes and drug response. To what extent different levels of iron stores contribute to the disparate ethnic responses to disease and treatments requires further investigation. A more complete knowledge of the aetiology of the differences in host inflammatory response could provide clinicians with the ability to optimize the therapeutic regimens tailored to the individual.
DECLARATION OF INTEREST
None.








