INTRODUCTION
Infectious intestinal disease (IID) is an important cause of morbidity and mortality throughout the world. The burden of illness is highest in developing countries with an estimated 6800 children dying on average each day from diarrhoeal diseases worldwide [Reference Farthing1–Reference Kaferstein3]. Deaths from IID in developed countries are less common, but still significant with an estimated 6400 deaths per year in the United States [Reference Mead4] and a significant morbidity. IID is usually self-limiting and does not generally lead to high costs to the particular individual. However, due to the large number of persons affected, the total costs at societal level can be considerable [Reference Roberts5–Reference Corso7]. A number of international studies have been performed to estimate the health burden and cost of illness using different approaches [Reference Roberts5, Reference Corso7–Reference Majowicz16]. In Malta, no estimates have been performed on the costs of IID at community level. In 2003, the Department of Health (Malta) embarked on a series of studies on the surveillance of IID. One component was a community-based population study. One of the objectives of this study was to estimate the burden of IID in terms of magnitude and cost of illness at the community level from a societal aspect. Such estimates are important to be able to compare with costs of possible different public health interventions and to assess the potential cost-effectiveness of specific interventions which would guide policy-making.
MATERIALS AND METHODS
To determine the burden and cost-of-illness due to IID, the number of cases, the number of resource units used and the cost per resource unit were obtained from the community population study. This was a retrospective cross-sectional study of a random sample of persons from among the Maltese population. A telephone interview was conducted between April 2004 and December 2005 and collected information on self-reported symptoms of IID at community level. Individuals who reported at least three episodes of diarrhoea (defined as loose stool) within 24 h or vomiting at least three times in 24 h, or suffered diarrhoea or vomiting with two or more additional symptoms in 24 h over the previous 28 days were defined as cases. Details on methodology are described in detail elsewhere [Reference Gauci17–Reference Gauci19].
Estimated number of cases
The period prevalence was obtained by dividing the number of cases by the total number of respondents. This was weighted to the age and gender structure of the Maltese population, and was applied to the population to obtain the expected number of cases per day and year in Malta. The number of IID episodes per person per year was obtained using the Poisson distribution to account for those cases that had more than one episode during the previous 28 days [Reference Gauci18].
Estimated resources used
Cases were asked questions regarding health-care use, use of medications, stool culture tests, personal costs, and loss of school or productivity. Since the use of resources generally depends on the illness severity [Reference Majowicz16], the self-perceived severity of illness, was thus categorized:
Mild cases. Cases with symptoms who felt slightly unwell but able to do all normal activities.
Moderate cases. Cases with symptoms who felt quite unwell but were still able to do most activities as well as those having to stay at home but who were able to get out of bed for limited activities.
Severe cases. Cases who had suffered the symptoms, who were confined to their home and being unable to do any of the usual activities as well as those who required hospitalization as a result of the illness.
Estimated costs of resources
The costs of resources comprised both direct and indirect costs. Costs were in Maltese lira (Lm) which is equivalent to €2.29. Direct costs included health-care provider (HCP) visits, hospitalization, stool culture testing, medications used directly for their illness and direct personal costs. Cases were asked for specific costs including telephone calls, special foods and drink, leisure items, new clothing, new bedding and cleaning materials. Indirect costs included missed work by the case or by other persons who cared for the case.
The average cost of health-care services and hospitalization costs were obtained from the fees legislation which was issued in 2004 [20]. The prices of medicines were obtained from the average selling prices obtained from a convenience sample of ten local pharmacies (of 207). The cost of analysis of a stool sample was estimated from the average total cost that private laboratories charge for testing in such cases, that is for bacteria (Salmonella, Campylobacter, E. coli, Shigella), rotavirus and protozoa.
To calculate accurately the indirect cost of IID due to lost productivity, the occupation of the case and of the person who missed work to care for a case was required. Since this information was not available for all the cases, the education level was used to calculate the salary under the assumption that cases work within their educational level. The education level was regrouped into six categories and the equivalent salary was obtained from information collected from the 2002 Health Interview Survey [21]. It was assumed there are 261 working days per year in calculating the salary per work day lost (public holidays were not taken in account since these vary from year to year). The mean days off work was calculated for all persons who were in employment at the time. For school days lost this was calculated for those who were attending school at the time of the interview.
The total costs were calculated from the above and reported per case of IID, overall per day and overall per year. Costing for IID was also calculated according to the grading of severity. Testing between two proportions was carried out using χ2 or Fisher's exact tests. Kaplan–Meier life tables were used for the calculation of the duration of illness since some of the cases were still symptomatic at the time of illness hence the exact duration of illness could not be calculated for all cases.
RESULTS
Rate of IID
The average monthly prevalence of 3·18% (95% CI 0·7–5·74) is the proportion of persons in the population who, at any point in time in the year, could report having had at least one episode of IID in the previous 28 days. Using Poisson distribution, a mean rate of 0·421 (95% CI 0·092–0·771) separate episodes of IID per person per year was estimated. When this figure is extrapolated to the general population, it corresponds to 164 471 (95% CI 35 941–301 205) episodes of IID per year in the Maltese Islands or an equivalent of 450 (95% CI 98–825) episodes of IID each day.
Severity of illness
Of the cases (n=99), 15% reported feeling a little out of the ordinary, 23% reported feeling slightly unwell but were able to do most activities (moderate IID), 20% reported feeling quite unwell but able to do all activities, 20% reported having to stay at home but were able to get out of bed for limited activities, 15% were confined at home and unable to do any usual activities whilst 4·8% required hospitalization.
The mean duration for which cases were unable to perform usual activities due to IID was 2·19 days (95% CI 1·94–2·45) with a mode of 2 days. The mean duration of time unable to engage in leisure activities was 2·36 days (95% CI 2·43–2·65) with a mode of 2 days.
The severity of illness was mild in 54·5% (n=54) of cases, moderate in 22·2% (n=22) of cases and severe in 23·3% (n=23) of cases. The majority of severe cases occurred in the 2–4 years age group whilst the mildest cases occurred in the 31–44 years age group (Fig. 1). Mild and severe cases were commoner among females whilst moderate severity illness was commoner in males.

Fig. 1. Category of severity of infectious intestinal disease (IID) illness per age group (n=99). ■, Mild; □, moderate;![]() , severe.
, severe.
Days taken off work and school
Of all the persons affected, 14% (n=14) missed paid employment due to the illness. The number of days off work ranged from 0 to 7 days. For those who had to take time off, the mean time was 1·86 days (95% CI 1·79–1·92) while the median and mode was 2 days. A number of cases did not require any time off work. This represents an average of 0·26 days of work lost per person suffering from IID.
The number of days lost varied with the severity of illness with 11·7% (n=11) of severe cases requiring an average of 5 days off work and none for mild cases.
For 4·4% (n=4) of cases, other persons took time off work to care for the case. The number of days required ranged from 1 to 5 days with a mean duration of 0·11 days (95% CI 0·22–0·002), which is equivalent to 0·0048 days of work lost per person suffering from IID. The days required off work varied with the severity of illness of the case. The median days off work was 3 days for both mild cases and severe cases and 1 day for moderate cases. The total number of days lost off work by the case was 0·26 and by the caregiver was 0·0048 which was equivalent to 0·2648 days lost per person with IID. When extrapolated to the population this is equivalent to 43 552 days lost each year in Malta due to IID. The lost productivity for the case was Lm 5·41 per day, and the lost productivity for the caregivers was Lm 8·02 per day for a total of Lm 13·43 per day per case of IID. This translates into a total of Lm 6044 of lost productivity due to IID per day in Malta or Lm 2 209 390 per year.
For 16% (n=16) of cases, school was missed because of the illness. The number of days missed ranged in duration from 0 to 6 days with a mean of 0·49 days (95% CI 0·37–1·45). This is equivalent to 0·0784 days of school lost per person with IID. The days missed varied according to the severity of illness with 12·5% of mild cases, 45·8% of moderate cases and 29·2% of severe cases of cases aged 3–16 years missing school.
Another 4·6% persons missed school because of another person with IID, for example siblings. The duration ranged from 1 to 5 days with a mean duration of 3·04 days (95% CI 1·39–4·64) and median and mode of 2 days. This is equivalent to 0·1398 days of school lost per person with IID, giving a total of 0·218 days of school lost per person with IID.
Health-care seeking behaviour
Of the cases, 55·5% (95% CI 46·68–64·32, n=55) sought the advice of a HCP by phone or by visiting general practitioner (GP). Of those who did not seek help, the majority (48%), did not do so because they felt it would be inadequate and that it would not make a difference to the outcome of their illness. The duration of illness influenced the health-seeking behaviour. The mean duration of illness in cases that sought help from a HCP was 7·61 days (95% CI 3·91–11·32) whilst in those who did not, it was less, 4·82 days (95% CI 2·99–6·65). The majority of those who contacted a HCP, consulted a doctor (95·6%, n=53) while 4·6% (n=2) consulted a pharmacist.
Almost half of the cases (48·4%, 95% CI 39·58–57·22, n=26) visited a HCP for their illness. Of these, 38·6% visited a GP, 14·2% visited the casualty department, 1·3% visited a health centre (public GP) and 3·5% visited another HCP.
A total of 89 visits to health professionals were recorded with a mean of 0·73 visits per case (95% CI 0·51–0·95). Of those attending the HCP, 61% were accompanied by someone, who in 4·4% of cases, had to make arrangements to accompany that person. For 23·9% of cases, a HCP visited the case at home. The commonest factor influencing the decision of the patient to visit the HCP was because they were suffering from diarrhoea. Age was significantly associated with health-care-seeking behaviour (P=0·011). Parents of children aged 0–4 years and those aged 12–18 years were found to consult more frequently than other age groups.
Of the cases identified, 6·35% (95% CI −2·10 to 14·80, n=3) required admission to hospital. The number of days of hospitalization ranged from 1 day to 35 days with a mean duration of 6·6 days (95% CI −0·69 to 13·89). Of cases who were admitted, 74·7% required a person to accompany the case, usually the mother for children. The maximum duration another person stayed with the case in hospital was of 5 days.
No complications were reported from any of the cases at the time of interview. However, no long-term follow up was performed. The costs incurred from health-care visits are listed in Table 1.
Table 1. Costs in Maltese liri (Lm) of consultation for IID with a health-care professional

Stool sample requests and submission
Of the cases who visited a HCP for IID, 11·2% (95% CI 1·79–20·61, n=6) were asked to submit a stool sample for analysis. This represents 5·4% of all the estimated cases suffering from IID. Of those who were asked for a sample, 14·5% were asked by the ward doctor or nurse, 73·9% by the hospital emergency doctor or nurse and 11·7% by a GP. The accuracy of these estimates is limited since they are based on a small number of patients since numerically of 99 cases, only two actually submitted a stool sample. In practice it has been shown that very few doctors actually ask patients to submit stools for analysis. The total costs incurred in sampling and analysis was Lm 40.
From the analysis of health-care-seeking behaviour and stool sample submission, the values can be extrapolated in that for every stool specimen submitted, there are 27 persons consulting a HCP and 50 persons suffering from IID in the community (Fig. 2).

Fig. 2. Estimated number of persons with infectious intestinal disease (IID) in the community, consulting a health-care provider (HCP) and submitting stools for analysis.
Extrapolating these estimates to the general population, it was calculated that there are 450 new episodes of IID each day in the community, of which 247 would consult a HCP and nine would submit stools for analysis (Fig. 3).

Fig. 3. Extrapolated number of persons with infectious intestinal disease (IID) in Malta each day, showing the number who visit a health care provider (HCP) and submit a stool sample.
Medication
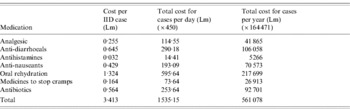
Of the cases with IID, 60·2% (51·58–68·82, n=60) reported taking some form of medication as a form of treatment for IID. The commonest medicinal taken was oral rehydration therapy (34·2%, 95% CI 25·77–42·63) which is usually purchased as an over-the-counter medicine from pharmacies. Alternative medications or remedies taken include chamomile tea, green tea, herbal tea, orange blossom water or tea with lemon. A higher proportion of females (63·9%) reported taking medicines than males in all age groups.
Using Kaplan–Meier life tables, cases who took medication were ill for an average of 7·03 days (95% CI 3·87–10·19) whilst those who did not take medications were ill for an average of 4·98 days (95% CI 2·73–7·22).
When testing by univariate and multivariate analysis for statistical significance, age, gender and duration of illness were not found to be significantly associated with medicinal use, both independently and when adjusted. The costs incurred in the required use of medicines are listed in Table 2.
Table 2. Costs in Maltese liri (Lm) incurred from use of medicines
Other costs
Costs of different items including special foods and drink, leisure items, new clothing, new bedding, prepaid school, prepaid leisure activities, other costs and costs related to phone calls amounted to Lm 0·189 per case which is equivalent to Lm 85·32 per day or Lm 31 183 overall in the community per year.
Total costs of IID
Direct costs included health-care professional visits and house calls, use of medicinals, costs of laboratory testing of stools and direct personal costs. Indirect costs were the costs involved in missed employment to the case and to caregivers. The total of indirect and direct costs per case are shown in Table 3.
Table 3. Total costs in Maltese liri (Lm) per case

The total direct and indirect costs incurred from IID per case of IID per day amounted to Lm 46·97, which is equivalent to a total cost of LM 21 138 per day or Lm 7 420 708 per year for the whole population.
Costs of IID according to severity
Since the resource units used differed according to the severity of illness, the resource units used by each case were classified according to the degree of severity. Table 4 lists the costs of resource unit used for the three categories of severity of illness. This is equivalent to Lm 6·05 per mild case, Lm 20·48 per moderate case and Lm 168·38 for a severe case.
Table 4. Cost in Maltese liri (Lm) of resources used according to severity of illness of IID in Malta

HCP, Health-care provider.
DISCUSSION
The traditional estimate of disease burden is based on the national surveillance system, which is reliant on notifications by doctors at GP and hospital level, and by laboratories. This reporting system does not cover those cases in the community who do not seek health-care services so that this study was the first step in estimating the true burden of this condition nationwide at community level. A cross-sectional methodology was chosen for the study in Malta since it is feasible, less costly and less time consuming [Reference Gauci17].
The high rate of IID reflects the significant burden of illness at community level. The rate was similar to that estimated for Ireland (4·5%, 95% CI 3·7–5·3), in 2000–2001 [Reference Scallan22–Reference Scallan24]. IID is usually self-limiting with the majority of cases being of mild and moderate severity so that the majority of the burden is experienced in the community. However, there were some severe cases of illness, often requiring admission to hospital for treatment. Age was related to the degree of severity of illness with children being more likely to have severe illness. This has been seen in other studies and is thought to relate to an under-developed immune system [Reference Monto and Koopman25, Reference Herikstad26].
The number of days taken off from work by cases and by other members of the family to take care of cases was considerable and was related to the severity of illness. The low rate (0·26 days) of missed work per case of IID in Malta was significantly lower than that found in other international studies. The UK study estimated 3 days for those consulting a GP and 2 days for those who did not [Reference Roberts5]. Rates fluctuated in different countries with 4·2 days reported in New Zealand [Reference Scott10, Reference Lake27]; 1·3 days in Australia [Reference Hellard8] and 1·8 days in Canada [Reference Majowicz16]. The lower number of days of work lost in Malta may be related to the very close family networks resulting in fewer people needing to take time off from work to care for other people in the family. An area of concern is that if symptomatic cases are returning to work in the early phase of illness, this may lead to transmission of illness to other work partners, especially for viral illness where person-to-person transmission usually predominates. The costs of time lost were calculated according to the education level. This assumption may not be correct in all cases since people may work in different jobs than expected by their educational level. This type of error can be reduced by asking for the actual work done and the income for the caregivers and the case, although this may be a sensitive question and responses may not be reliable especially for self-employed persons and those in part-time employment.
Time lost from education was seen to be considerable among those affected and may have had considerable impact especially if it occurred at a crucial time in the year, for example during examination days. However no value has been placed upon this subjective element here.
GPs are an important contact point for cases of IID. Although there is a well-established public GP service in Malta many patients would prefer to consult their own family doctor. This Maltese study has revealed a higher rate of consultation with GPs than studies in other countries [Reference Roberts5, Reference Van den Brandhof14]. This is very important for surveillance initiatives since it is clear that almost half of the cases of IID were visiting a GP. Hence GPs can be a target point for surveillance and for encouraging submission of stool samples for analysis. The study in Ireland, with similar rates of IID as in Malta, showed a lower consultation rate of 29·2% [23]. Different health-care systems, accessibility and nationwide health consciousness may affect the consultation rates. Although admission to hospital occurred in a low percentage of cases, the costs of this are very high. Stool sample requests are quite low in Malta when compared to 6·9% in Ireland [23]; 27% in the United Kingdom [Reference Wheeler28] and 34% in Norway [Reference Kuusi29]. Similarly, submission rates of samples may be low, hence the high rate of unspecified foodborne illness reported in national statistics via the routine surveillance system may be expected. The findings of this study will help in the interpretation of national surveillance data especially given consultation rates and stool submission rates for IID.
The use of medicines for the treatment of IID also contributed to a significant cost to the community which is related to the duration and severity of illness. In severe cases, the cost was very high due mainly to hospitalization costs. Cases of IID, use up resources that could be used for other illnesses or other patients. Thus, these costs can reflect the opportunity costs given scarce hospital resources.
This study has shown that IID represents a substantial disease burden in the community with significant costs, of which the health-seeking costs were by far the largest component followed by lost productivity. The cost values reported in this study were substantial, yet they are likely to be an underestimate of the true cost. Some components were not included in the analysis due a to lack of information, e.g. lost leisure time, lost quality of life, lost work which is usually unpaid, psychological factors, lost schooling, chronic sequelae, public health surveillance and investigation and loss to industry. This study did not include the more than one million tourists who visit Malta every year especially in summer so the burden of illness and hence cost to the health service described here is not complete.
It is clear from the findings of this study that interventions to reduce the prevalence of community IID should consider sources of sporadic cases in addition to sources of outbreaks since they add considerably to the burden and costs. These significant additional costs justify efforts to take the necessary measures to control this disease.
ACKNOWLEDGEMENTS
We thank our colleagues working at the Disease Surveillance Unit, within the Department of Public Health for their professionalism and diligence in carrying out the interviews and the respondents for their cooperation. We also thank Dr Malcolm Micallef, Director Public Health; Dr Shannon Majowicz, Epidemiologist at Health Canada and Dr Elaine Scallan, Epidemiologist at CDC Foodborne Diseases Active Surveillance Network (FoodNet) for their invaluable help.
DECLARATION OF INTEREST
None.









