INTRODUCTION
Candida spp. are common inhabitants of the mucosal membranes of the gastrointestinal tract of mammals. The prevalence of Candida spp. colonization in the gastrointestinal tract ranges from 25–50% in healthy individuals, but C. albicans may reach up to 80% in hospitalized patients [Reference Hazen, Howell, Baron, Landry, Jorgensen and Pfaller1–Reference Fridkin and Jarvis3].
Candida spp. can cause invasive as well as non-invasive infections and have been implicated in 10–15% of all nosocomial infections [Reference Eggimann, Garbino and Pittet2, Reference Fridkin and Jarvis3]. Bloodstream infection (BSI) is a life-threatening invasive disease associated with significant mortality and morbidity. Data from North American and European surveillance programmes of hospital-acquired bacteraemia have revealed that Candida spp. are the fourth most common cause, accounting for 8–10% of nosocomial BSIs [Reference Wisplinghoff4–Reference Puzniak7]. C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis are the most frequently encountered causative agents of candidaemia. More rarely, C. krusei, C. lusitaniae, C. guilliermondii, and C. rugosa are detected. The mortality associated with Candida spp. BSI is consistently high, with estimated figures between 40% and 50% in adults and 20% in children [Reference Diekema5]. Candidaemia can vary from a self-limiting infection to a severe clinical picture leading to sepsis and multiple organ failure [Reference Pfaller, Pappas and Wingard8, Reference Viudes9].
The frequency of isolation and the relative proportion of C. albicans to non-albicans Candida spp. are highly divergent in different countries and hospitals and knowledge of their distribution are crucial for the choice of empirical antifungal treatment. Fluconazole and amphotericin B are the most widely used drugs worldwide but the side-effects associated with amphotericin B limits its use. The azoles are much safer but resistance has been seen towards azoles in Candida [Reference Xess10]. The objective of this retrospective investigation was to characterize the incidence and epidemiology of candidaemia in adults and paediatric patients hospitalized in a large university-affiliated hospital over a 12-year period and to determine the fluconazole susceptibility pattern of some isolates. The epidemiology of candidaemia in adult intensive-care units (ICUs) was additionally investigated as critically ill patients are at particular risk for candidaemia because of their debilitated condition and frequent need for invasive procedures [Reference Davis, Vazquez and McKinnon11, Reference Bassetti12].
MATERIALS AND METHODS
Setting and study design
Uludağ University Hospital is a tertiary-care 800-bed hospital that includes six ICUs (resuscitation, surgical, burn, stroke, neurosurgery, cardiovascular), haematology and oncology clinics, and a renal transplantation unit. Candidaemia cases were identified through the surveillance of all blood culture isolates in the hospital microbiology database from 1996 to 2007. The incidence of candidaemia was calculated as the ratio of total number of patients to 1000 admissions and to 10 000 patient-days. The 12-year survey was divided into two 6-year periods (1996–2001 and 2002–2007) for ease of comparison.
Definition of candidaemia
Blood cultures are taken routinely when patients deteriorate and/or have fever. A nosocomial episode of candidaemia occurring 48 h after admission was defined as at least one positive blood culture yielding Candida spp. during a single hospitalization. Subsequent positive cultures were defined as new episodes only if at least 12 weeks had lapsed since the previous isolate and at least 14 days of treatment with an antifungal agent had been completed with resolution of symptoms.
Identification of organisms
Clinical isolates were detected using an automated continuous monitoring blood culture system (Bactec 9240, Becton Dickinson Inc., USA). No special media for fungal blood cultures was used. Blood culture bottles positive for yeasts following a Gram stain were cultured on Emmon's modified Sabouraud dextrose agar (SDA 2%) and inhibitory mould agar (IMA) and incubated for 24–72 h at 35°C. The isolates were identified by standard procedures (germ tube production, morphology on cornmeal Tween-80 agar, and/or chromogenic agar) and analysis of biochemical patterns by ID 32C (bioMérieux, France).
Antifungal susceptibility testing
Routine antifungal susceptibility tests are not performed in our hospital. To determine fluconazole susceptibility, 214 isolates of Candida spp. other than C. krusei (one isolate per infection episode), were randomly selected. Of these isolates 92, 86 and 45 were recovered in the years 1996–1998, 2000–2001, and 2007, respectively. Fluconazole susceptibility was determined using the CLSI M27-A2 reference method for broth microdilution antifungal susceptibility testing of yeasts [13]. The control strains C. parapsilosis (ATCC 22 016) and C. krusei (ATCC 6258) were run in parallel with the test isolates. Minimum inhibitory concentrations (MICs) were read after 48 h of incubation at 35°C and interpretative breakpoints were determined according to the CLSI guidelines [13]. Resistant, susceptible dose-dependent, and susceptible to fluconazole were defined as MICs ⩾64 μg/ml, 16–32 μg/ml, and ⩽8 μg/ml, respectively.
Statistical analysis
Statistical analysis was performed with SPSS software, version 13.0 for Windows (SPSS Inc., USA). Categorical variables were given with number and percent values, and χ2 analysis was used to test for differences in the proportions of categorical variables between two or more groups. Fisher's exact test (two-tailed) was used in 2×2 tables instead of a χ2 test when the sample size was small. A value of P<0·05 was accepted as statistically significant.
RESULTS
Candidaemia incidence
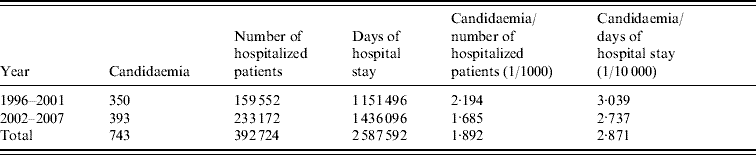
The number of annual admissions ranged from 18 823 in 1996 to 42 987 in 2005, and over the 12-year period, 743 episodes of candidaemia (457 adult, 286 paediatric patients) were detected in 392 724 patients on a total of 2 587 592 patient-days (Table 1). The annual incidence of candidaemia ranged from 1·4 to 3·6 (average mean 1·9) cases/1000 hospital admissions and the incidence per 10 000 patient-days per year ranged from 2·4 to 5·4 (average mean 2·9). The annual incidence was almost constant during the study period except for 1996 (Fig. 1) when it was significantly higher in comparison to other years, with 3·559/1000 admissions and 5·374 for 10 000 patient-days (P<0·001). A significant reduction of incidence was also found between the two study periods regarding 1000 patients admission and 10 000 hospital days (P<0·001) (Table 1).
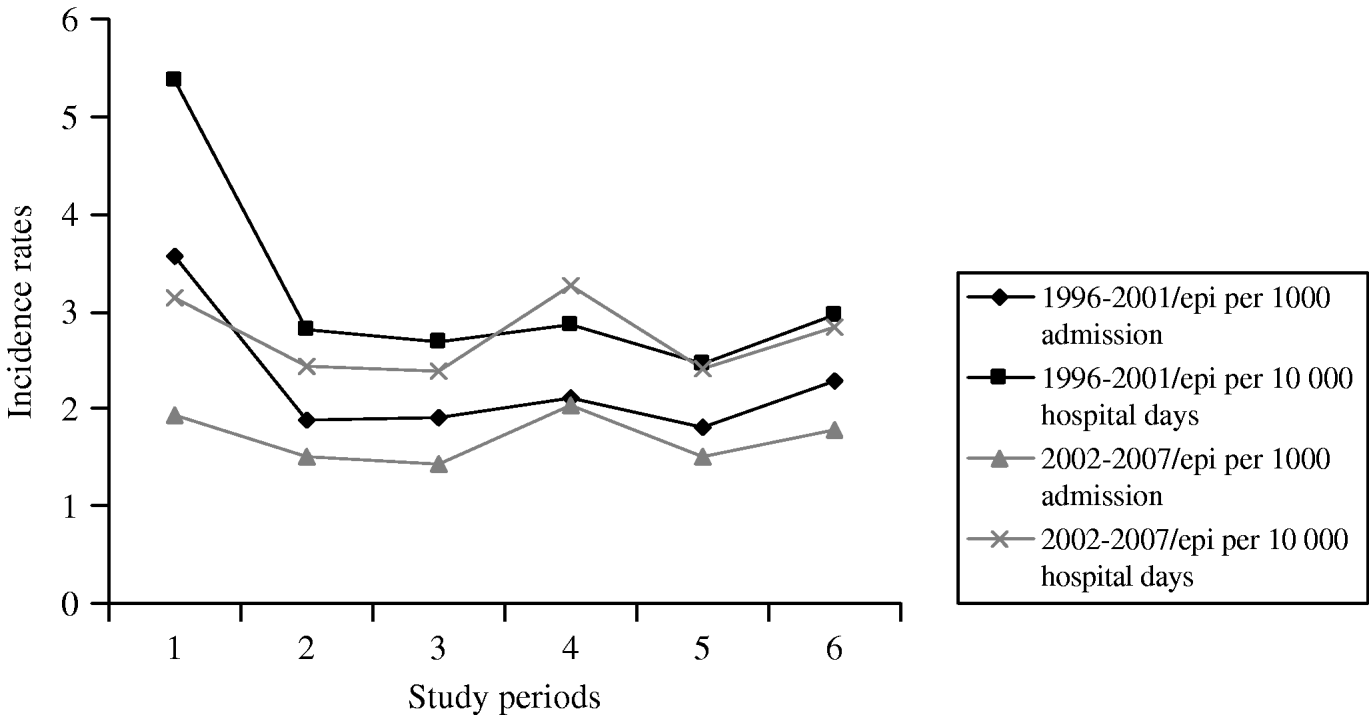
Fig. 1. Incidence of candidaemia during 1996–2007.
Table 1. Average rates of candidaemia during the two study periods
Epidemiology of candidaemia
The species distribution in adult patients and children aged <18 years during the two study periods is shown in Table 2. In the first period (1996–2001), there were no episodes of candidaemia involving more than one species of Candida, but 18 patients (4·6% of all patients) harboured two different species in the second period (2002–2007). In total, 411 isolates were obtained from 393 patients. C. albicans remained the predominant species, 39% and 50% of all isolates recovered, in the first and second period, respectively, and C. parapsilosis was the second most common. Compared to the first period, a significant increase in C. albicans isolates causing candidaemia was observed in the second period, mainly because of a decrease in C. parapsilosis isolates in adult patients and C. krusei isolates in children (P<0·05). There were no definitive differences in C. glabrata isolation between the two study periods both in adult patients and children, and significantly increased isolation of C. parapsilosis was observed in children during the second period (P<0·05). C. kefyr was predominantly isolated from adult patients, whereas C. lipolytica was isolated from the paediatric group.
Table 2. Distribution of Candida spp. isolated from blood during the two study periods
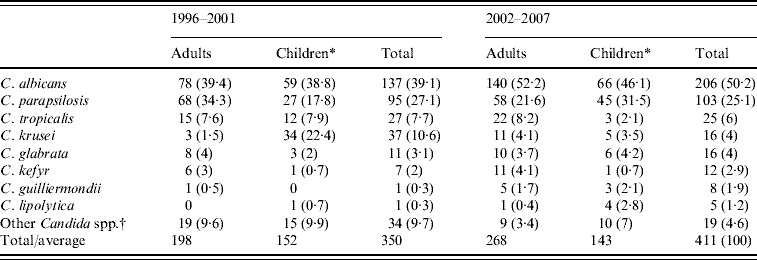
Values are number (%).
* Patients aged <18 years.
† C. pelliculosa, C. lusitaniae, C. zeylanoides, C. inconspicua, C. dubliniensis, and non-specified non-albicans Candida spp.
Table 3 details the species distribution of candidaemia in the adult ICUs; 36% of all episodes occurred in adult patients (166/457) hospitalized in the medical and surgical ICUs. C. parapsilosis was the most common species in these patients with an approximate rate of 40% in the first period, whereas in the second period, C. albicans was the most common aetiological agent. Thus, C. albicans increased by 20·9% (P=0·003) during 2002–2007 compared to 1996–2001, whereas C. parapsilosis decreased by 15·2% (P=0·0015).
Table 3. Candida spp. in adult intensive care unit patients
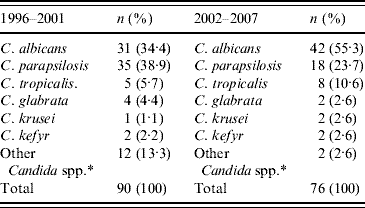
* C. lipolytica, C. lusitaniae, C. guilliermondii, and non-specified non-albicans Candida spp.
Fluconazole susceptibility
The MICs for the two control organisms tested by the reference method in all the sets of experiments consistently agreed with those from the CLSI reference results, confirming both the reproducibility of the results and the correct drug concentrations. The fluconazole susceptibility of the 214 Candida spp. isolates tested is presented in Table 4; the overall resistance rate was 1·9% (MIC ⩾64 μg/ml). All C. albicans and C. tropicalis isolates were susceptible to fluconazole (MIC ⩽8 μg/ml) whereas 2·6% and 14·3% of C. parapsilosis and C. glabrata isolates, respectively, were resistant. Reduced susceptibilities (16–32 μg/ml) were obtained in some C. parapsilosis, C. glabrata, C. kefyr and C. pelliculosa isolates. No significant changes in susceptibility to fluconazole were seen over time in the C. albicans, C. parapsilosis and C. tropicalis strains tested between 1996 and 2007 (data not shown). Due to the limited number of isolates, similar comparisons with other Candida spp. were not calculable.
Table 4. Fluconazole susceptibility of Candida spp.
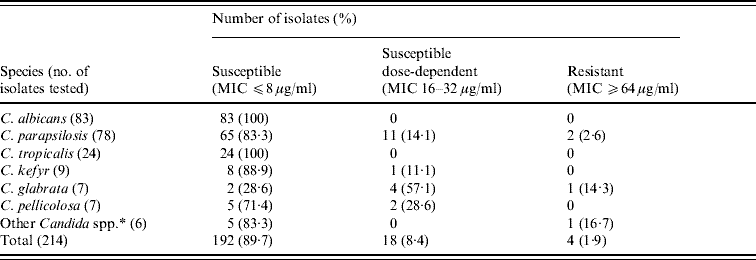
MIC, Minimum inhibitory concentration.
* C. guilliermondii (3 isolates), C. dubliniensis, C. lusitaniae and C. zeylanoides (1 isolate each).
DISCUSSION
Invasive candidiasis is the most frequent life-threatening fungal disease, and candidaemia represents 20–30% of all invasive cases. According to the criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infection Group, a single positive blood culture is considered sufficient to prove invasive fungal infection [Reference de Pauw14]. Several recent studies have reported a steady increase in the incidence of Candida BSIs over the last two decades [Reference Pfaller and Diekema15]. This increase is multifactorial in origin and reflects advances in medical and surgical technology. Improved chemo- and immunosuppressive therapy, transplant medicine, and intensive-care technology have decreased the mortality of many life-threatening diseases but have also led to an increase in patients vulnerable to infections. In the USA, Candida spp. are now the fourth most common cause of nosocomial BSIs, and in many countries these organisms account for 5–8% of all blood culture isolates [Reference Wisplinghoff4, Reference Davis, Vazquez and McKinnon11, Reference Pfaller and Diekema15].
Surveys performed at a single hospital have tremendous value for therapeutic decision-making and prevention measures at the local level. As only a single report documenting the incidence and spectrum of organisms responsible for candidaemia in a Turkish hospital has been published [Reference Yapar16], we performed a hospital-wide survey that included all hospital units. Although this study was restricted to a single hospital, a large sample size (2·5 million patient-days) was attained. The incidence of candidaemia (1·9/1000 patients and 2·9/10 000 patient-days) in our centre is higher than the rates reported from centres in the USA and other European countries, including the above-mentioned report from our country [Reference Wisplinghoff4, Reference Tortorano6, Reference Pfaller and Diekema15, Reference Yapar16]. Morgan [Reference Morgan17] cites the incidence of candidaemia from different countries to be between 0·2 and 2·8/1000 admissions and 0·3 and 1·14/10 000 patient-days. The incidence rates most similar to those found here (above 1·5/10 000 patient-days) have been reported from Italy, Spain, Belgium, Brazil, and Taiwan [Reference Tortorano6, Reference Almirante18–Reference Chen21]. It is well documented that the frequency of isolation of Candida spp. varies widely in different countries and hospitals. Numerous factors contribute to this such as differences in patient demographics and comorbidities, as well as medical practices, especially the use of long-term vascular catheters and antibacterial and antifungal prescribing patterns. We speculate that the high incidence of candidaemia in our tertiary-care teaching hospital of 800 beds is probably due to the large patient population at risk for infection. The surveillance system (NNIS) in the USA showed a clear association between the incidence of candidaemia and the number of hospital beds and/or the academic affiliation [Reference Banerjee22] and corroborated the European Confederation of Medical Mycology survey [Reference Tortorano6].
Much higher rates are observed when paediatric age groups are evaluated separately [Reference Rangel-Frausto23]. An earlier study of paediatric patients in our hospital, found the incidence of candidaemia to be 5·1/1000 admissions [Reference Çelebi24], and this undoubtedly impacts on the total incidence observed in this survey. Our hospital has a large-capacity neonatal ICU that cares for most of the premature infants and newborns in the region.
As seen in Table 1, the significant reduction in the incidence of Candida infections is marked in the second period (2002–2007) of the study. Improvements in the application of infection control precautions, more conscious and appropriate use of parenteral nutrition, improvements in the care of central venous catheters, limited use of the broad spectrum antibiotics since 2002, and widespread infectious diseases consultation are factors considered to have played a role in this decrease, and it is believed that this decreasing trend will continue.
The frequency of diagnostic test ordering, especially of blood cultures, and the type of blood culture systems employed might also impact on the incidence rates in laboratory-based surveillance [Reference Pfaller and Diekema15, Reference Horvath25]. Good laboratory practice and clinical cooperation and training, a single type of blood culture system during the study period, and physicians accustomed to frequently ordering blood cultures at our institute might be other reasons for this high incidence.
More than 17 species of Candida have been reported to be the aetiological agents of candidaemia in humans, but most invasive infections are attributed to C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis. In both study periods, C. albicans remained the predominant species, but the 13% increase (P=0·006) in the frequency of isolation of this species in the second period appears to be an unusual finding. Although most countries are experiencing a decrease or stable incidence of C. albicans BSIs over time, one report from the USA has noted a rise in the frequency of isolation of C. albicans [Reference Pfaller26]. The increased isolation of C. albicans might be a consequence of the decreased isolation of C. parapsilosis and C. krusei in adults and paediatric patients, respectively. Nevertheless, C. albicans remains the dominant species causing BSIs throughout the world with low frequency (37%) in Latin America to 70% in Norway [Reference Pfaller and Diekema15]. We found the rate of C. albicans to be 45% over the 12-year study period, which is similar to frequencies reported from the USA and some European countries [Reference Hajjeh27, Reference Cuenca-Estrella28].
The proportion of Candida bloodstream isolates due to non-albicans species appears to be on the increase [Reference Pfaller and Diekema15]. This change is believed to be related to the introduction of fluconazole prophylaxis, but the reasons for the variability in the frequency of the different species from diverse studies remain controversial [Reference White29, Reference Kunova30]. C. glabrata has undoubtedly emerged as an important opportunistic pathogen in the USA, and virtually every US-based survey has shown C. glabrata to rank second to C. albicans as a cause of BSI, accounting for 20–24% of all Candida BSIs [Reference Pfaller and Diekema15]. In contrast, C. glabrata is much less common as a cause of BSI in most other countries, and the lowest frequency of C. glabrata as a cause of BSI has been reported in Latin America, where only 4–7% of Candida BSIs are attributed to this species [Reference Colombo20]. In our study, the total frequency of C. glabrata was found to be 3·5% over the 12-year period, which is similar to the surveys in Latin America and Turkey [Reference Yapar16]. The reasons for such marked variation in the frequency of C. glabrata as a cause of BSIs are unclear but may include exposure to azoles, patients' age, underlying disease, geographic location, or other, unknown factors [Reference Pfaller and Diekema15]. In our centre, haematopoietic stem cell transplantation has not yet been performed, and liver transplantation has only recently begun (in 2008). Prophylactic fluconazole use has not been widespread in this hospital, and the lower frequency of C. glabrata might be attributed to this. Throughout this survey, we used the same blood culture system and a longer incubation period (72 h), which is thought to be efficient for the isolation of C. glabrata; therefore, the possibility of false-negative culture results can be discounted [Reference Horvath25].
Similar to C. glabrata, the isolation of C. krusei can be related to fluconazole usage. This species has emerged in blood and marrow transplant recipients receiving fluconazole prophylaxis but fluconazole exposure alone does not explain the reported increase in infections caused by this species [Reference Iwen31]. In most of the surveys, C. krusei accounts for 2–4% of all Candida BSIs [Reference Pfaller and Diekema15]. Significant differences were observed here between the two periods with higher frequencies of isolation of C. krusei recorded in the first period (P<0·05). Although elevated frequencies of C. krusei have been reported in cancer patients, our high rate was clearly due to the paediatric age group (Table 2) where an outbreak in the first study period was thought to be responsible for this high rate.
C. parapsilosis is an exogenous pathogen that may be found on skin rather than mucosal surfaces. This species is notorious for its ability to form biofilms on catheters, for nosocomial spread by hands and for persistence in the hospital environment [Reference Pfaller and Diekema15]. Here, C. parapsilosis was found to be the second most common isolate after C. albicans, accounting for 27·1% and 25·1% of all Candida BSIs, in the first and second period, respectively. C. parapsilosis is also well-known for causing infections in infants, neonates and ICU patients. In a report from Turkey, the ratio of C. parapsilosis was found to be 37·2% in paediatric patients, which is also similar to our rates for the second period [Reference Bakir32]. In contrast, the frequency of C. parapsilosis isolation significantly decreased in adult patients hospitalized in ICUs and clinical wards (Tables 2 and 3). Parallel to the decrease of incidence in the second period, the lower isolation of C. parapsilosis also reflects improvements in the infection control strategies at our centre. The total frequency of C. tropicalis was 6·8% over the 12-year study period, which is similar to rates in different countries and hospitals except in Latin America (∼20%) [Reference Pfaller and Diekema15].
Fluconazole and amphotericin B are the most widely used drugs worldwide for Candida infections. The side-effects associated with amphotericin B limits its use and detection of resistance to amphotericin B by the CLSI M27-A2 method has been problematic due to the very narrow range of MICs obtained [Reference Pfaller and Diekema15]. Thus, fluconazole susceptibility testing is considered more important in epidemiological studies. Moreover, decreased susceptibility to fluconazole may precede decreased susceptibility to voriconazole [Reference Pfaller and Diekema15]. Clinical laboratories performing antifungal susceptibility testing of fluconazole against Candida spp. can reliably use these results as a surrogate marker for voriconazole [Reference Pfaller33]. Here, ∼90% of isolates were susceptible to fluconazole and none of the C. albicans and C. tropicalis isolates exhibited in vitro resistance confirming the rarely seen fluconazole resistance in C. albicans isolates outside of AIDS patients with recurrent oropharyngeal candidiasis [Reference Rex34, Reference St-Germain35]. According to population-based and sentinel surveillance programmes, C. glabrata and C. parapsilosis organisms resistant to fluconazole have been noted in ∼10% and 1% of BSI isolates, respectively, with the exception of higher frequencies (40% and 15%) from Sweden [Reference Pfaller and Diekema15].
In conclusion, candidaemia and invasive candidiasis are persistent health problems. Epidemiological studies have revealed differences between countries and centres and emerging species that may vary geographically in terms of frequency of isolation. It is therefore essential that laboratories identify clinical isolates of Candida to the species level. The choice of appropriate antifungal treatment based on local epidemiological data is very important, and epidemiological changes indirectly reflect antifungal policy and efficacy of catheter care and infection control measures.
DECLARATION OF INTEREST
None.







