Methicillin-resistant Staphylococcus aureus (MRSA) infections are now among the most frequently seen antibiotic-resistant infections in many areas in the world [Reference Graffunder and Venezia1–Reference Clancy3]. Moreover, MRSA rates continue to increase worldwide according to current data supplied by ongoing surveillance monitoring such as the National Nosocomial Infections Surveillance System (NNIS) and the European Antimicrobial Resistance Surveillance System (EARSS). Indeed, the incidence of MRSA in intensive-care units (ICUs) in the USA reached 60% in 2003 showing an 11% increase compared with the previous time interval 1998–2002 [2]. On the other hand, the prevalence of MRSA infection shows considerable variation between countries, even in different areas of the same country and in different hospitals in the same area [Reference Asensio4–6]. According to EARSS data, the prevalence of MRSA in hospitals in some Northern European countries such as The Netherlands is low (<4%) but exceeds 25% in Southern Europe, the UK and Ireland [6].
The prevalence of MRSA in Turkey has been reported to be between 45% and 70% depending on the study population [Reference Alp5, Reference Topeli, Unal and Akalin7, Reference Esel8]. Turkey joined EARSS in 2005, but has been participating in the Antibiotic Resistance Surveillance and Control in the Mediterranean region (ARMed) project and data for 2003 to 2005 were added to the EARSS database. MRSA infections showed a significant decrease from 43% in 2003 to 36% in 2006 despite the fact that the data were limited to only the 14 participant hospitals in Turkey [6].
In the present study, the rates of nosocomial MRSA infections were determined over a 3-year period in a tertiary-care teaching hospital in Turkey. Further, the influence of institution-specific and patient-associated factors on the rates of infection was investigated.
The tertiary-care teaching hospital has 250 beds and three ICUs: anaesthesiology & recovery (six beds), neurosurgical (five beds) and cardiovascular (four beds) ICUs. Each patient's room has a sink with unmedicated soap for hand washing. Dispensers of hand antiseptic solutions are placed only in high-risk areas, not in all patients' rooms, and colour posters emphasizing the importance of hand washing are displayed in these areas. Moreover, an educational programme for health-care personnel is carried out by the infection control team.
Medical records of patients hospitalized between January 2004 and December 2006 were analysed retrospectively. Nosocomial S. aureus-infected cases were defined as those individuals who grew S. aureus from culture of specimens collected at least 48 h after admission. MRSA-infected cases (case patients) were those who had nosocomial S. aureus that showed methicillin resistance, while those with methicillin-sensitive S. aureus were classified as nosocomial MSSA cases (controls). Infections were defined using the Centers for Diseases Control and Prevention (CDC) criteria.
Clinical and epidemiological data were collected by a specialist in infectious diseases and the Infection Control Committee Nurse. Information about associated factors for development of nosocomial MRSA infections was collected from patients' files and included prolonged hospitalization (>21 days), hospital ward, surgery, diabetes mellitus, malignancy, renal insufficiency, obesity, trauma, surgery, mechanical ventilatory assistance, previous antibiotics, and insertion of vascular catheter, urinary catheter, or nasogastric tube.
S. aureus was identified by Gram stain, catalase reaction, tube coagulation test, and Api-Staph test (bioMérieux, France). Methicillin resistance was determined by Kirby–Bauer disk diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS) criteria [9].
Continuous variables were compared using the independent-samples t test and categorical variables were compared using the χ2 test or Fisher's exact test for association. Differences were considered statistically significant when P<0·05. Statistical calculations were performed using SPSS for Windows 11.0 (SPSS, USA). Forward stepwise multiple logistic regression was performed to identify the independent predictors of outcome, determine factors associated with the presence of methicillin resistance in nosocomial S. aureus infections as the dependent variable and all factors significant on univariate analyses as covariates. Variables with significant collinearity were omitted from the analysis.
A total of 13 511, 13 775, and 17 757 patients were hospitalized in 2004, 2005, and 2006, respectively. Rates of ventilator use in the anaesthesiology & recovery ICU were 0·79 (2004), 0·67 (2005) and 0·71 (2006), rates in the neurosurgical ICU were 0·43 (2004), 0·45 (2005), and 0·41 (2006), and rates in the cardiovascular ICU were 0·50, 0·71, and 0·51, respectively for each of the three years.
During the study period 265 nosocomial S. aureus infections were identified in 231 patients, and 66·4% were due to MRSA. One hundred and thirty-eight patients (60%) were male and ranged in age from newborn to 93 years with a mean of 51·3 years (s.d.=22·2). The incidence rate of nosocomial S. aureus decreased from 5·6 cases/1000 admissions in 2004 to 5·0 cases/1000 admissions in 2006 (P=0·52). Correspondingly the incidence of MRSA infection increased linearly with years from 2·4 cases/1000 admissions in 2004, to 3·77 cases/1000 admissions in 2006 (χ2 for linear trend 3·850, P=0·05). The number of MRSA infections rose from 39/88 patients (44·3%) in 2004 to 62/80 (77·5%) in 2005, and this was maintained in 2006 (75/97, 77·3%) (χ2 for linear trend 21·797, P<0·001). MRSA prevalence was highest in the anaesthesiology & recovery ICU and general surgery unit and lower in the paediatrics unit (P=0·001) (Table 1). The most common clinical presentation was pneumonia which was present in almost half (49·4%) of patients (Table 2).
Table 1. Comparison of the prevalence of methicillin resistance in ICUs and non-ICUs
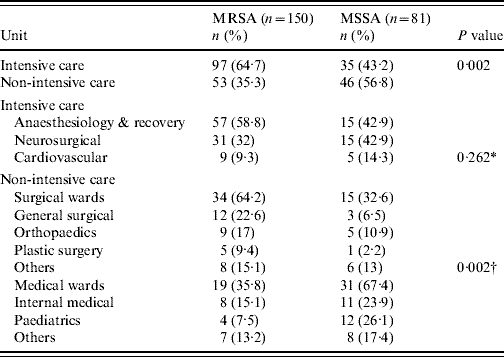
ICU, Intensive-care unit.
* Comparison between ICUs.
† Surgical vs. medical wards.
Table 2. Nosocomial MSSA and MRSA infections in hospitalized patients by site of infection, 2004–2006
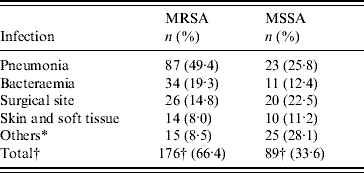
* Omphalitis, central nervous system, urinary tract, in-dwelling catheter, osteomyelitis and septic arthritis.
† Number of nosocomial MRSA and MSSA infections.
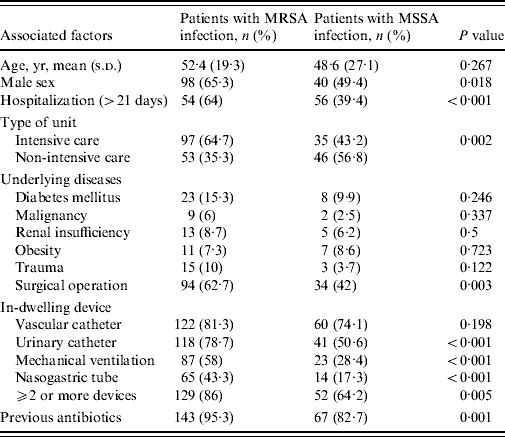
Table 3 shows the univariate analysis for the main potential associated factors for MRSA infections and identified the statistically significant variables as prolonged hospitalization, type of hospital ward, surgical operation, urinary catheterization, mechanical ventilation, male sex, presence of nasogastric tube and previous antibiotics usage. Results from the multivariate analysis showed that, when considered together, prolonged hospitalization (OR 3·982, 95% CI 2·235–7·094, P<0·001), mechanical ventilation (OR 3·052, 95% CI 1·666–5·590, P<0·001), surgical operation (OR 2·032, 95% CI 1·102–3·748, P=0·023), and male sex (OR 2·000, 95% CI 1·081–3·699, P=0·027) were significantly and independently associated with MRSA infection.
Table 3. Univariate analysis of associated factors for methicillin resistance in nosocomial S. aureus infections
Our study revealed the overall mean proportion of MRSA in nosocomial S. aureus infections in a Turkish tertiary-care hospital was 66·4%. Although MRSA rates reported by EARRS for 14 participating hospitals in Turkey were often at the higher end in European countries, our rate proved to be even higher. However, similarly high rates have been reported from another hospital in Turkey (66%) and Brazil (60·2%) [Reference Esel8, Reference Guilarde10]. Since MRSA was first recognized in 1960, rates of MRSA have increased worldwide in the ensuing decades with some fluctuations [Reference Asensio4, 6, Reference Guilarde10]. An interesting finding from the present study was that in spite of stable rates of overall S. aureus nosocomial infections, there was a statistically significant increasing trend in the proportion of MRSA infections, We believe that one of the important contributing factors to this issue is the limited financial resources available for implementation of infection control practices in our hospital such as the provision of hand-washing solutions in each patient's room and contact isolation of MRSA-infected patients. Although there was a significant increase in the number of hospitalized patients in our institution, a similar trend was not evident in the rate of ventilator use in ICUs. There was a concomitant increase in the incidence rate of nosocomial MRSA infection from 2·4 cases/1000 admissions in 2004 to 3·77 cases/1000 admissions in 2006. However, the retrospective design of the study is not ideal to establish the incidence rate. Shitrit et al. [Reference Shitrit11] found that with implementation of active surveillance and contact isolations, the incidence of nosocomial MRSA bacteraemia decreased from 0·74 to 0·37 cases/1000 admissions. Similarly, a recent study reported that the implementation of surveillance programmes resulted in a decrease in MRSA infection rates from 6·1 to 4·1 infections/1000 census days [Reference Clancy3].
In a recent study [Reference Asensio4], the prevalence of MRSA was highest in patients admitted to ICUs and, this is confirmed by our finding of significantly higher rates in the anaesthesiology & recovery ICU and general surgery unit and lower in the pediatrics unit. MRSA prevalence was found to be lowest for bacteraemia as a primary site of infection and highest for respiratory tract infections in that study. Here, in all sites of infection MSSA was similar to or more frequently isolated than MRSA with the exception of nosocomial S. aureus pneumonia which was caused predominantly by MRSA. This may have been due to more frequent use of mechanical ventilation in the ICUs as the multivariate analysis showed that this was the second most significant factor associated with acquisition of MRSA infection. This is a very important finding that could help us to guide the initial antimicrobial therapy in patients with nosocomial pneumonia.
Of the associated factors revealed by the univariate analysis, prolonged hospitalization, mechanical ventilation, surgical operation, and male gender proved to be independent risk factors for hospital-acquired MRSA infection as has been reported in other studies [Reference Graffunder and Venezia1, Reference Topeli, Unal and Akalin7, Reference Guilarde10, Reference Selvey, Whitby and Johnson12]. Our data indicate that MRSA infection increased almost fourfold where patients were hospitalized >3 weeks. The reason for prolonged hospitalization may be the severity of the primary illness and not always related to MRSA infection. Surgical operation increased MRSA infections by twofold, in keeping with other published studies [Reference Graffunder and Venezia1, Reference Coello13]. The influence of gender on MRSA rates is controversial. Like us, Selvey et al. [Reference Selvey, Whitby and Johnson12] found males predominated in MRSA cases but another study disagreed with this finding [Reference Manzur14]; therefore this is an area clearly requiring further investigation.
Patients with MSSA were used as a control group but a limitation of the study was the lack of a group of patients without S. aureus infection. Selection of the MSSA group as controls may have magnified the risk of individual factors and falsely identified risk factors [Reference Harris15]. Nevertheless, several factors proved to be independently associated with MRSA in this Turkish population and this has significant implications for the selection of empirical treatment or restriction of antibiotics and strategy for control measures.
DECLARATION OF INTEREST
None.





