INTRODUCTION
Encephalitis is a complex syndrome with many potential infectious, post-infectious and non-infectious causes. Infectious causes, including viruses, bacteria, fungi, and parasites, represent more than half of the known causes of encephalitis, with herpes simplex virus in adults and varicella-zoster virus in children playing a significant role [Reference Mailles and Stahl1]. However, the role of different aetiological agents causing encephalitis is changing [Reference Glaser and Bloch2]. While measles, mumps, and rubella were historically important causes of encephalitis, their importance has diminished, largely as a result of immunization campaigns [Reference Koskiniemi3]. In more recent history, pathogens that were formerly rare or unknown, such as West Nile virus, Nipah virus, Australian bat lyssavirus, influenza A(H5N1), and enterovirus 71 [Reference Gubler4–Reference Wang8], have emerged as causes of encephalitis. Some of these pathogens result in high case-fatality rates or severe sequelae, which makes encephalitis an important public health issue [Reference Mailles and Stahl1]. A rise in encephalitis cases may signal the emergence of novel pathogens with outbreak potential [Reference Huppatz9], introduction of a pathogen to a new area such as West Nile virus to North America, or mutation of a previously known pathogen, which underscores the importance of understanding the aetiology and epidemiology of encephalitis-causing aetiologies.
Reviews of hospitalization records in the USA [Reference Khetsurani, Holman and Anderson10], Australia [Reference Huppatz11], UK [Reference Davison12], and France [Reference Mailles, Vaillant and Stahl13] have consistently identified a large proportion of encephalitis cases with unknown aetiology, ranging from 60% to 80%. This has motivated efforts to characterize the range of potential aetiological agents through active surveillance and clinical studies [Reference Mailles and Stahl1, Reference Glaser14, Reference Granerod15]. However, the cause of encephalitis remains undetermined in a significant proportion of patients despite a range of diagnostic tools available, including clinical examination, isolation, serological testing and molecular analysis [Reference Mailles and Stahl1].
Several arthropod-borne viruses (arboviruses) endemic to Canada are known causes of encephalitis. West Nile virus is one such pathogen, which was first detected in New York in 1999 [16] and rapidly spread across North America, with activity first detected in Canada in 2001 [Reference Drebot17]. Other arboviruses, such as St Louis encephalitis virus and Western equine encephalitis virus, have caused sporadic epidemics in Canada [Reference Artsob18], while, with increased diagnostic capacity and awareness, our understanding of the possible public health importance of the California serogroup and Cache Valley viruses is emerging [Reference Meier-Stephenson19, Reference Pabbaraju20]. Eastern equine encephalitis virus is an emerging veterinary health problem in Canada [Reference Chénier21], with potential to become a public health threat. Travel-associated infections due to arboviruses such as tick-borne encephalitis and Japanese encephalitis viruses have also been recognized in Canada [Reference Artsob and Spence22].
Despite the occurrence of numerous arboviruses in Canada that cause neuroinvasive disease, West Nile virus is the only arbovirus under national surveillance. This raises the potential for underdiagnosis of other arboviral infections, which may be contributing to an unrecognized burden of encephalitis in Canada.
To better define the case load and epidemiology of encephalitis in Canada, we reviewed national hospitalization discharge diagnoses over a 15-year period and assessed the association of demographic and geographical factors with different aetiological categories of encephalitis. Using spatial and temporal statistics, we then identified seasonal clusters of hospitalizations that may implicate underlying arboviral agents for many undetermined aetiologies of encephalitis.
METHODS
Data sources
Data on hospital discharge diagnoses were obtained from the Hospital Morbidity Database (HMD), 1994–2005, and Discharge Abstract Database (DAD), 2006–2008, held by the Canadian Institute for Health Information (CIHI; www.cihi.ca). The HMD and DAD use the International Classification of Diseases, 9th revision and its Clinical Modification as well as the 10th revision (ICD-9, ICD-9-CM, ICD-10). The data are subject to CIHI's data quality framework to ensure completeness and accuracy of records. Codes for encephalitis-related diagnoses were obtained through a review of similar studies [Reference Khetsurani, Holman and Anderson10, Reference Huppatz11]. Encephalitis-associated hospitalizations were extracted from the database based on ICD code (Table 1), and recoded into ICD-9 using CIHI conversion tables. An encephalitis-associated hospitalization was defined as a hospitalization for which at least one of the ICD encephalitis codes appeared among the first three discharge diagnoses, i.e. the primary discharge diagnosis and two additional diagnosis codes (of 17 possible codes).
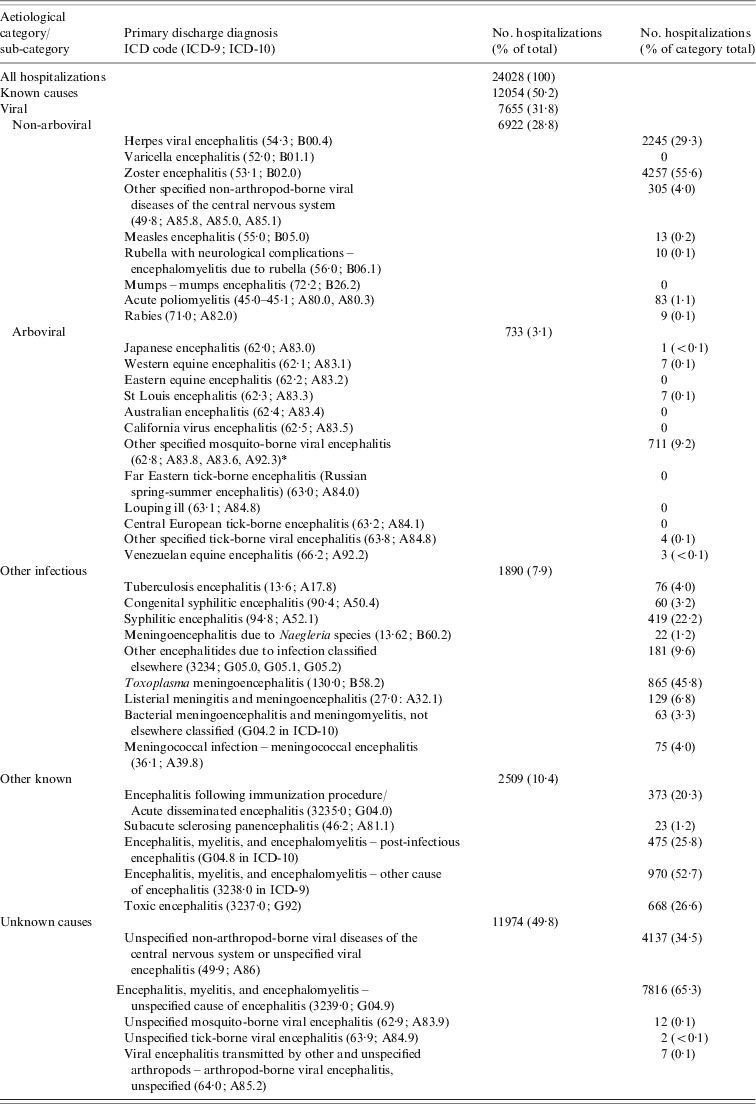
Table 1. International Classification of Disease (ICD) codes for encephalitis-associated hospitalizations by aetiological category

* Includes Mosquito-borne West Nile viral encephalitis (A92.3 in ICD-10).
Coding inconsistencies consisting of missing decimal points in some ICD-9 datasets were detected that prevented the distinction of varicella encephalitis diagnoses from other non-encephalitis varicella diagnoses; therefore, the code for varicella was excluded from analysis. Similarly ICD-9 codes for subacute sclerosing panencephalitis were indistinguishable from acute pharyngitis and were excluded. ICD codes were classified into five categories: viral (comprised of arboviral and non-arboviral), other infectious (bacterial or parasitic), other known (post-infectious, post-immunization, toxic or other) and unknown (unspecified cause). Viral, other infectious and other known causes constituted the ‘known’ category. The Atlantic provinces (New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland, Labrador) and Northern territories (Northwest Territories, Yukon, Nunavut) were grouped, respectively, for analysis. Duplicates, defined as two or more hospitalizations in a given year having the same health card number, were excluded. Similarly, hospitalizations with province of diagnosis different from province of residence (based on postcode) were considered as travel-related and excluded from the analysis.
Analysis
Data were analysed using SAS Enterprise Guide (EG) version 4.1 (SAS Institute Inc., USA). Hospitalization rates were calculated based on annualized census population estimates available from Statistics Canada (www.statscan.gc.ca). Age, sex and region were included in multivariable logistic regression models for grouped (blocked) data to assess the association between these factors and the different aetiological categories of encephalitis. Annual differences in mean hospitalization rates for all causes of encephalitis and seasonal differences for unknown aetiologies were assessed using a negative binomial regression model. A P value <0·05 was considered statistically significant.
A discrete Poisson model in SaTScan™ v. 8.0 [Reference Kulldorff23, Reference Kulldorff24] was used to analyse spatial and temporal clustering of hospitalizations associated with encephalitis of unknown and mosquito-borne viral aetiologies in Canada in 2003, which was chosen because it was a peak year in the early stages of the West Nile virus epidemic in Canada [25]. With the discrete Poisson model, the number of cases in each location is Poisson-distributed. Under the null hypothesis, the expected number of cases in each area is proportional to its population size, or to the person-years in that area [Reference Kulldorff24]. The scan statistic adjusts for the uneven geographical density of the background population. The space–time scan statistic is defined by a cylindrical window with a circular geographical base and with height corresponding to time. A cluster with P value <0·05, based on maximum likelihood, is considered statistically significant. In the analysis, number of cases and population size were defined at the geographical unit of forward sortation area (FSA), which represents the first three digits of the postal code. FSA population and centroid coordinates were obtained from Statistics Canada. Coordinate data were generated using the reported postal code from the 2001 census returns with interpolated boundaries generated for each FSA; as a consequence, there was more than one polygon associated with some FSAs. In such instances, the polygon with the largest area was used to define the FSA centroid. Cases were stratified by date of hospital admission. A 7-day scanning window was selected to detect clusters of cases. Independent analyses were conducted for encephalitis hospitalizations of unknown aetiology and for hospitalizations attributed to an endemic mosquito-borne viral encephalitis, which includes West Nile virus (ICD-10: A92.3), Western equine encephalitis (ICD-9: 62.1; ICD-10: A83.1), St Louis encephalitis (ICD-9: 62.3; ICD-10: A83.3), and other specified mosquito-borne encephalitis (ICD-9: 62.8; ICD-10: A83.8) (Table 1). The cluster centroid location and radius, output from the analysis, were used to map clusters of hospitalizations in ArcGIS v.10 (ESRI, USA).
RESULTS
Aetiological categories
During the 15-year period from 1994 to 2008 there were a total of 24028 encephalitis-associated hospitalizations in Canada, with an annual average rate of 5·4/100000 population. Unexplained aetiologies accounted for the largest proportion of encephalitis-associated hospitalizations in Canada, comprising 49·8% (2·57/100000 population) (Table 2). Viral aetiologies accounted for 31·9% of all hospitalizations (1·64/100000 population). Of the viral encephalitides, the primary causative agents were zoster, accounting for 4257 (17·7%) hospitalizations, and herpes virus, accounting for 2245 (9·3%) hospitalizations. Zoster encephalitis was unevenly age- and sex-distributed, with 80% of zoster hospitalizations occurring in adults aged ⩾65 years and 63% occurring in women. Arboviruses, including tick- and mosquito-borne pathogens, were responsible for 733 (3·1%) of total encephalitis hospitalizations (Table 3).
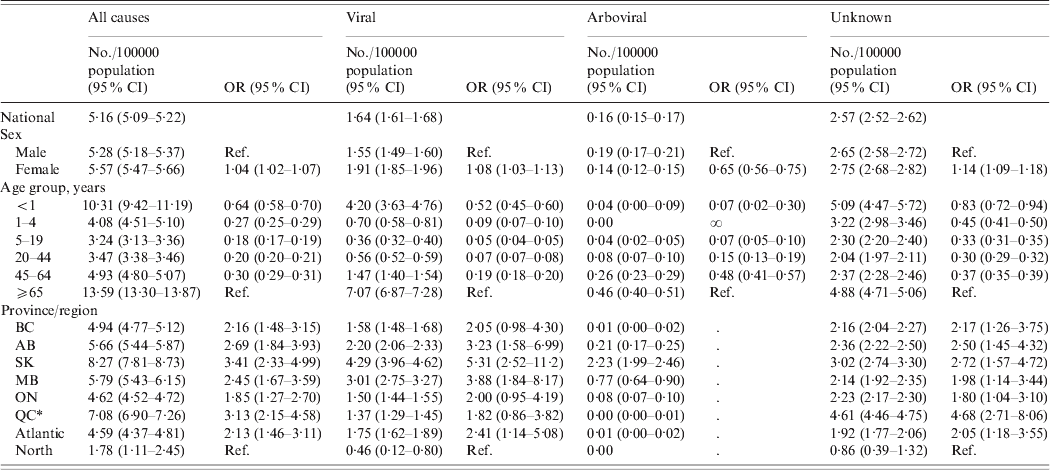
Table 2. Encephalitis-associated hospitalizations by disease category, sex, age and province/region, Canada, Hospital Morbidity Database, 1994–2005 and Discharge Abstract Database, 2006–2008

OR, Odds ratio; CI, confidence interval; BC, British Columbia; AB, Alberta; SK, Saskatchewan; MB, Manitoba; ON, Ontario; QC, Quebec.
* QC figures represent hospitalization rates from 1994 to 2005.
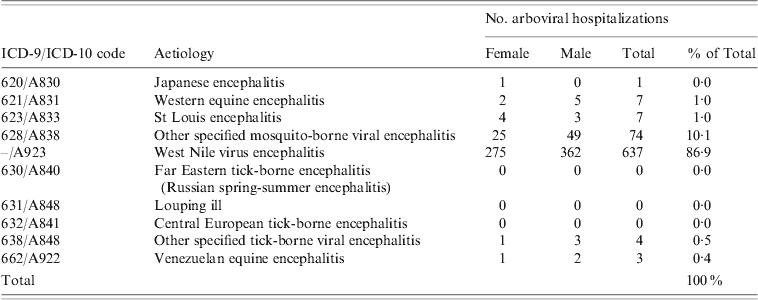
Table 3. Encephalitis-associated hospitalizations with arboviral ICD codes, Hospital Morbidity Database, Canada, 1994–2005 and Discharge Abstract Database, 2006–2008

Seasonality of hospitalizations
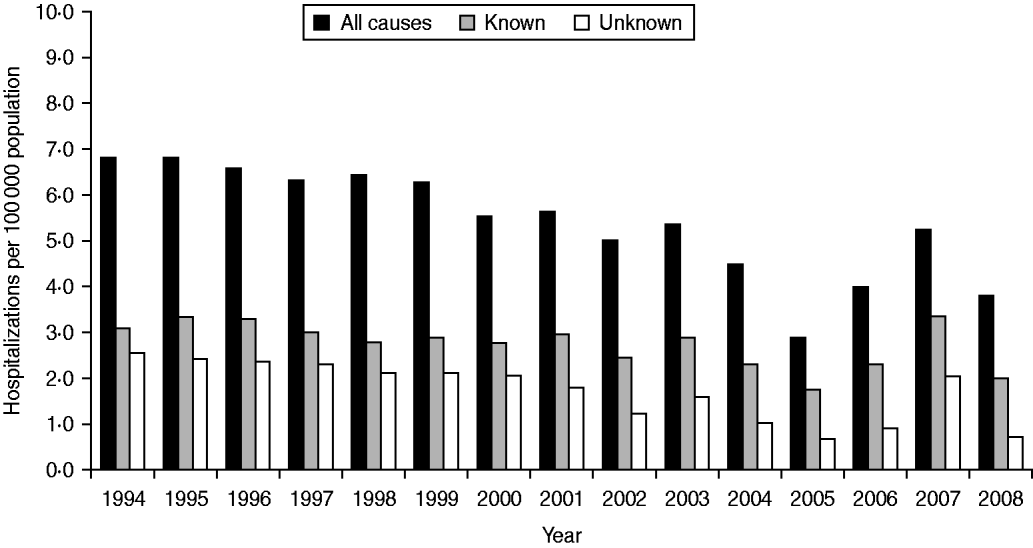
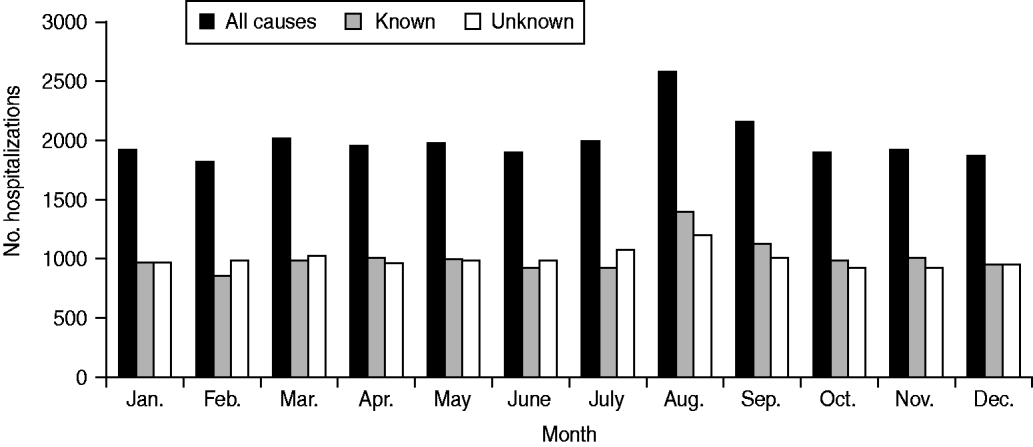
Rates of hospitalization for all causes, known causes and unknown causes did not differ significantly over the study period (Fig. 1). Monthly average hospitalization rates peaked in August for both known and unknown encephalitis aetiologies (Fig. 2). Seasonal differences were observed in mean rates of hospitalization associated with encephalitis of unknown aetiology for all provinces (P < 0·05 in negative binomial regression). In general, rates of hospitalization were significantly higher in summer (June–August) and autumn (September–November) than in spring (March–May), except for Quebec where differences in seasonal rates of hospitalization were not significant. Elevated hospitalization rates in the winter months (December–February) were also observed in British Columbia, Saskatchewan and Quebec.

Fig. 1. Encephalitis-associated hospitalizations per year for all causes, known aetiologies and unknown aetiologies, 1994–2008.

Fig. 2. Encephalitis-associated hospitalizations per month for all causes, known aetiologies and unknown aetiologies, 1994–2008.
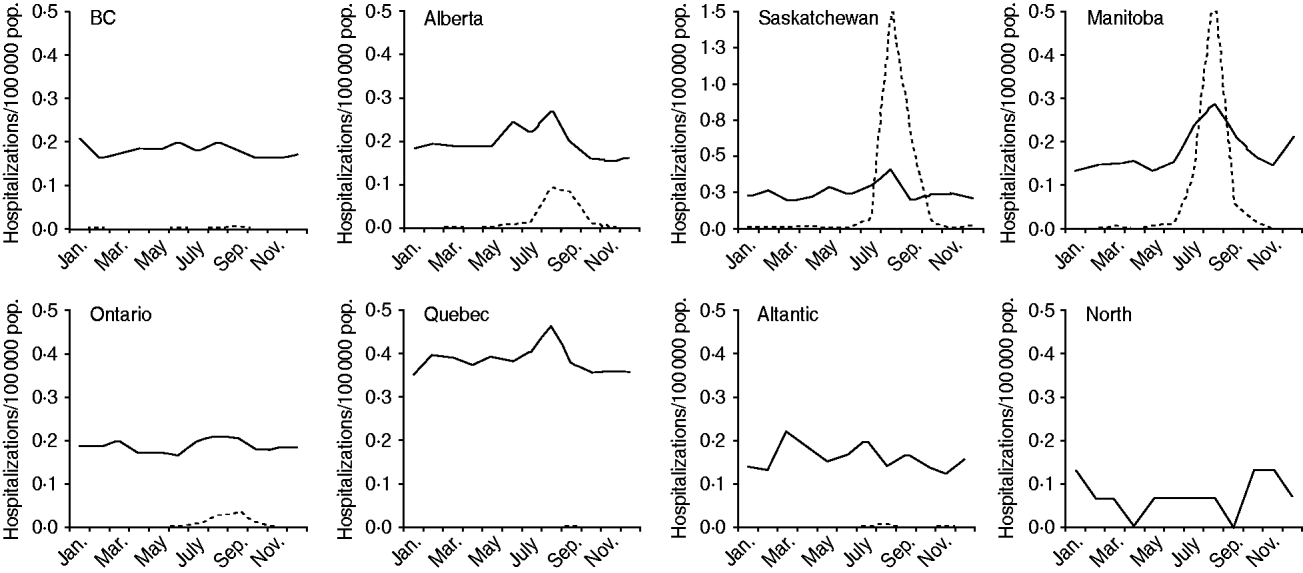
Arboviral encephalitis hospitalizations displayed a seasonal pattern, with increased hospitalization rates observed in all provinces/regions in summer and autumn compared to winter and spring, although this difference was only statistically significant for Ontario (P < 0·05 in negative binomial regression). Increased hospitalization rates associated with unknown encephalitides in summer and early autumn paralleled the rise in arboviral encephalitis hospitalizations in many provinces where arboviral infections would be expected to occur (Fig. 3).

Fig. 3. Monthly rates of hospitalization associated with encephalitis of unknown (solid line) and arboviral (dotted line) aetiologies, by province/region, 1994–2008.
Hospitalizations by age, sex and province/region by aetiological category
At the national level, women had a marginally higher risk of encephalitis-associated hospitalization than men [odds ratio (OR) 1·04, 95% confidence interval (CI) 1·02–1·07]; however, this trend differed according to aetiological category (Table 2), with the higher incidence of zoster encephalitis in women largely accounting for the difference.
For viral aetiologies, women had higher risk of hospitalization than men (OR 1·08, 95% CI 1·03–1·13). This was mainly due to a larger number of hospitalizations associated with zoster encephalitis for women compared to men (1·40/100000 population vs. 0·84/100000 population, respectively). In contrast, for other infectious aetiologies, women had lower odds of hospitalization than men (OR 0·65, 95% CI 0·60–0·71). This was primarily due to higher number of hospitalizations due to Toxoplasma encephalitis in men than women (0·31 vs. 0·15/100000 population, respectively). HIV infection was listed in the first three discharge diagnoses for 56·1% of all Toxoplasma encephalitis hospitalizations. Women had higher odds of hospitalization than men for encephalitis of unknown aetiology (OR 1·14, 95% CI 1·09–1·18).
There were age-related differences in hospitalizations associated with encephalitis in all aetiological categories (P < 0·05 in logistic regression). Viral aetiologies were associated with highest risk in adults aged ⩾65 years and infants (Fig. 4). This was similar to the pattern observed for hospitalization due to encephalitis of unknown aetiology, for which older adults and infants were at highest risk, with elevated risk also for children aged 1–4 years. Other known non-infectious (i.e. post-infectious and post-immunization) causes of encephalitis were associated with highest risk in adults aged ⩾65 years and children aged 1–4 years, while other (non-viral) infectious agents mainly affected adults aged ⩾65 years.

Fig. 4. Encephalitis-associated hospitalizations by age group for all causes and different aetiological categories, 1994–2008.
There were province-related differences in hospitalizations associated with encephalitis in all aetiological categories (P < 0·05 in logistic regression) (Table 2).
While at the national level women, infants and adults aged ⩾65 years had the greatest odds of hospitalization associated with encephalitis of unknown aetiology, there were significant age × province and sex × province interaction effects (P < 0·05) that suggest regional differences in the epidemiology of unclassified encephalitis hospitalizations. This was explored further using cluster analysis.
Epidemiology of hospitalizations associated with arboviral aetiologies
West Nile virus was the leading causative agent of arboviral encephalitis nationally, accounting for 86·9% of the 733 arboviral encephalitis hospitalizations (Table 3). Of the remaining arboviral hospitalizations, 10·1% were diagnosed as ‘other specified mosquito-borne viral encephalitis’ and a small number of cases (3%) as other mosquito-borne aetiologies, such as Japanese encephalitis, Western equine encephalitis, St Louis encephalitis and Venezuelan equine encephalitis, or ‘other tick-borne viral encephalitis’ (Table 3). The highest rates of hospitalization associated with arboviral encephalitis were in the Prairie provinces: Saskatchewan, Manitoba and Alberta (Table 2). At the national level, arboviral hospitalizations were significantly associated with age and sex. Men were at higher risk of arboviral infection than women, and adults aged ⩾65 years were at greater risk than younger age groups, consistent with the expected trends for West Nile virus.
Space–time cluster analysis
The distribution of hospitalizations in Canada in 2003 associated with encephalitis of unknown and mosquito-borne viral aetiologies is shown in Figure 5. Three significant space–time clusters of unclassified encephalitis hospitalizations were detected in eastern Canada (end of February to end of August), the central Prairies (end of June to mid-September) and northern British Colombia (mid-May to early October) (Fig. 6). Two significant clusters of arboviral encephalitis hospitalizations were detected, in southern Ontario (early to late September) and the central Prairies (early August to early October). Notably, the Prairie clusters of arboviral and unclassified encephalitis hospitalizations overlapped both in space and time, highlighting the potential for a common aetiology.

Fig. 5. Distribution of hospitalizations associated with encephalitis due to unknown and mosquito-borne viral aetiologies in Canada, 2003. AB, Alberta; BC, British Columbia; MB, Manitoba; NB, New Brunswick; NL, Newfoundland; NS, Nova Scotia; NU, Nunavut; NWT, Northwest Territories; ON, Ontario; PEI, Prince Edward Island; QC, Quebec; SK, Saskatchewan; YK, Yukon.

Fig. 6. Space–time clusters of hospitalizations associated with encephalitis due to unknown and arboviral aetiologies, 2003.
DISCUSSION
Encephalitis, or inflammation of the brain, is a clinical syndrome associated with mortality or morbidity that often leaves survivors with permanent neurological impairments, from seizures, to cognitive problems, to motor or sensory deficits. In clinical practice in Canada there is no standard case definition and encephalitis is broadly recognized by initial symptoms of headache, fever, chills, nausea and vomiting, progressing to mental confusion and somnolence and possibly profound coma, with variable neurological findings [Reference Mandell, Doublas and Bennett26]. A wide array of infectious agents can cause encephalitis, including a number of arboviral and emerging zoonotic pathogens. Given the broad clinical definition of encephalitis, investigations to determine the aetiology of encephalitis cases should take into account several factors, such as outbreak history (e.g. are similar cases occurring in neighbouring territories), patient's age, sporadicity of cases, history of arthropod or animal bite, history of travel, and severity of disease. This information may help guide the types of tests requested.
Despite a growing arsenal of diagnostic tests available, the aetiological agent of encephalitis was not classified in half of the patients hospitalized in Canada between 1994 and 2008. While this statistic reflects similar findings in other countries [Reference Khetsurani, Holman and Anderson10–Reference Mailles, Vaillant and Stahl13], it highlights the need for a better understanding of the aetiology of encephalitis in order to improve diagnostic capacity for this complex syndrome. Diagnosis of the aetiological agent of encephalitis is important for early treatment, particularly for bacterial infections, and appropriate public health intervention. However, in addition to the non-specific clinical presentation of encephalitis, diagnosis is challenged by poor sensitivity of available tests for known causes, failure to test for uncommon pathogens, and the lack of tests for as yet unrecognized infectious agents [Reference Glaser and Bloch2]. In addition, antibody-associated causes are increasingly recognized in encephalitis [Reference Granerod27].
By investigating the epidemiology of unclassified encephalitis hospitalizations in Canada we were able to identify similarities to viral and post-infectious causes (that are associated with higher risk in younger and older age groups), with significant differences apparent between provinces/regions of the country. While this analysis masks smaller-scale variation and clustering, the finding of geographical variation in the epidemiology of unknown causes of encephalitis suggests the contribution of multiple aetiological agents. Three key findings support the idea that undiagnosed arboviral illness may be contributing to the burden of unclassified encephalitis in some regions of Canada. First, the summer/early autumn peaks in hospitalizations for encephalitis of unknown aetiology from Alberta in the west to Quebec in the east closely mirrored peaks in known mosquito-borne viral encephalitides. These were consistent with an epidemiological pattern expected of encephalitides caused by other arthropod-borne viruses that are driven by ecological determinants. Second, using space–time statistics, we identified a cluster of unclassified encephalitis hospitalizations that overlapped geographically and temporally with a cluster of West Nile virus encephalitis hospitalizations, suggesting a common aetiology between clusters. The cross-jurisdictional occurrence of these clusters tends to rule out possible ‘administrative’ differences in reporting, diagnostic testing strategies, etc. in different jurisdictions as causes of geographical variation in reporting of cases. Third, additional space–time clusters of hospitalizations for encephalitis of unknown aetiology occurred in British Columbia and eastern Canada. These occurred from spring to early autumn so an arboviral cause cannot be ruled out although clearly other agents may have contributed.
Several factors may have affected our estimates of the overall and cause-specific burden of encephalitis, including removal of duplicate records that would exclude the possibility of recurrences. Similarly, the analysis was conducted excluding ICD-9 codes in which coding inconsistencies were noted, including the code for varicella, a leading cause of encephalitis. Additional coding errors in hospitalization records may have been present that could potentially influence the estimates of encephalitis burden. Inclusion of the first three discharge diagnosis codes, including the primary discharge diagnosis, may also have excluded some true encephalitis-associated hospitalizations.
Failure to detect arboviral agents of encephalitis may relate to multiple factors, including physician awareness and diagnostic capacity. A national West Nile virus surveillance system was implemented in Canada in 2000, consisting of human, animal (birds and horses), and mosquito surveillance. The national response plan included development of appropriate laboratory testing capacities, and generation of information for clinicians, public health authorities and the general public [Reference Artsob, Buck and St28]. West Nile virus infection became nationally notifiable in Canada on 1 June 2003 [29]. During the initial outbreak of human cases in 2002, only a few Canadian laboratories had flavivirus ‘in house’ testing procedures employed to screen for viral antibody, and all confirmatory assays were performed by the National Microbiology Laboratory (NML). Commercial diagnostic kits were available in provincial public health laboratories from 2003 onwards, with supplementary testing available at the NML upon referral from provincial laboratories. The observation of overlapping clusters of unclassified and arboviral encephalitis hospitalizations early on in the spread of West Nile virus in Canada may therefore relate to regional variations in diagnostic capacity at this stage [Reference Drebot17]. Another possible reason may be that diagnostic standards to establish a confirmed West Nile virus case definition were not met in many patients, who are often found with positive titres that do meet the rigorous criteria to differentiate past virus exposure.
Given the nature of the national hospitalization datasets used in the analysis we were unable to link laboratory testing information with hospital patient records to verify which diagnostic tests were performed. This would have helped to clarify the findings of certain arboviral encephalitides, since no cases of St Louis encephalitis or Western equine encephalitis are known to have occurred during the study period. Cross-reactivity of antigens in assays (e.g. St Louis encephalitis antibody cross-reactivity with dengue antigen), misinterpretation of persistent IgG titres as more recent infections (e.g. for Western equine encephalitis) or disease classification on the basis of clinical findings alone may help to explain these results.
Further laboratory testing information for the cases in our study would also help to differentiate between illnesses that were undiagnosed as a result of lack of testing, versus the occurrence of novel aetiological agents. The close geographical concordance of disease clusters with unclassified and arboviral aetiologies in the Prairie provinces, which closely relates to the distribution of the regional principal vector of West Nile virus, Culex tarsalis [Reference Curry30] supports the hypothesis that West Nile virus probably contributed to a proportion of the undiagnosed cases. However, other infectious agents may potentially contribute to the burden of encephalitis identified in our study, such as California serogroup viruses [Reference Meier-Stephenson19], which may be an under-recognized cause of human disease in Canada; Eastern equine encephalitis, which has recently emerged in Canada [Reference Chénier21]; as well as Powassan and other mosquito- and tick-borne viruses [Reference Artsob18].
Since arboviral encephalitis usually represents the ‘tip of the iceberg’ with respect to arboviral cases, improved diagnosis of other cases with neurological involvement may help to better demonstrate the full range of arboviruses circulating in Canada that may cause encephalitis. Further information on the aetiology of encephalitis in Canada could therefore be made through the application of newer techniques to testing of specimens from cases of encephalitis, and inclusion of antigens from other arboviruses isolated in Canada that may cause human disease, such as Flanders virus which is common in Canadian mosquitoes, as well as Silverwater, Northway, Cache Valley and California serogroup viruses. The occurrence of unclassified encephalitis in infants and young children in Canada, which mirrors the epidemiology of several non-West Nile virus arboviral agents, underscores the potential diversity of arboviral diseases that may occur in Canada and the need for greater awareness of and vigilance for diseases that may emerge as a result of ongoing climate and environmental changes, or through introduction by international travel or trade.
Further study is needed to define ecological correlates with spatial clusters of undiagnosed encephalitis in order to identify potential underlying aetiological agents. Spatial studies incorporating vector species distributions and other environmental characteristics may help to identify the attributable fraction of undiagnosed encephalitis that can be linked to arboviruses, and identify risk areas for targeted surveillance and public health intervention. However, encephalitis is not a nationally notifiable condition in Canada although it is reportable in many provinces; consequently, diagnostic testing and reporting are not well standardized and vary by location. Inclusion of encephalitis as a nationally notifiable condition could help to standardize case definitions and diagnostic testing procedures. Syndromic surveillance for encephalitis could form a component of an arbovirus surveillance system through education of physicians in risk areas. Such surveillance could also provide a means for early detection of emerging infectious diseases. Importantly, the system would need to address the limitations inherent to passive surveillance, which relies on clinicians considering diagnosis of an arboviral disease and obtaining appropriate diagnostic tests, and on reporting of laboratory-confirmed cases to public health authorities [Reference Lindsey31].
Given the health burden associated with encephalitis for the individual, its impact on vulnerable populations (particularly seniors and children), the economic burden associated with healthcare and rehabilitation, and the societal cost associated with care of survivors, a better understanding of the aetiology of encephalitis in Canada is needed to enhance diagnostic capacity and define appropriate public health action.
ACKNOWLEDGEMENTS
This study was funded by the Public Health Agency of Canada.
DECLARATION OF INTEREST
None.