INTRODUCTION
A recent increase in the number of human cases of rodent-borne Puumala hantavirus (PUUV) in northwest Europe has highlighted the possibility of its endemic transmission in Great Britain (GB), particularly given the ubiquity of the reservoir host (the reservoir host, Myodes glareolus, is absent from Northern Ireland). The transmission dynamics of PUUV are complex, involving interactions between environment, tree biology, rodent population cycles and human risk behaviour. Owing to the increasing number of human cases in Europe, and the non-specific clinical features of human infection, there is the potential for unrecognized cases in GB. This paper discusses the ecological influences of PUUV transmission in northwest Europe, and assesses whether similar ecological circumstances exist in GB that might permit localized PUUV-associated human cases.
In Europe, PUUV is the most common and important serotype [Reference Clement1, Reference Maes2], spread by its rodent host Myodes (formerly Clethrionomys) glareolus (bank vole) which is abundant in northwest Europe [Reference Keyaerts3]. PUUV causes nephropathia epidemica (NE) in humans, a relatively mild form of haemorrhagic fever with renal syndrome (HFRS), one of the two main clinical presentations of hantavirus disease. The transmission dynamics of PUUV differ between Scandinavia and northwest Europe (e.g. France, Belgium, Germany), but are related in both cases to the population dynamics of M. glareolus.
In Scandinavia, M. glareolus populations behave cyclically, mainly due to predator–prey cycles [Reference Olsson4]. In northwest European countries, several major NE outbreaks [Reference Clement1, Reference Escutenaire5–Reference Hofmann9] occurring almost simultaneously in recent years, have been associated with increases in M. glareolus population numbers, and these increases have been associated with peak years in tree crop production of beech mast (Fagus sylvaticus) (known as ‘mast years’), and also oak acorns (Quercus sp.) [Reference Escutenaire5, Reference Mailles7, Reference Piechotowski10–Reference Clement14]. This association has been described for M. glareolus populations in GB [Reference Ashby15–Reference Smyth17] with some studies suggesting that, although there may be no regular multi-annual cycles as there are in Scandinavia, sporadic multi-annual fluctuations can occur in addition to the annual population fluctuations, according to season and habitat [Reference Alibhai and Gipps18–Reference Southern and Lowe20]. Other studies argue that there is evidence to support the hypothesis of a marginal true cycle, complete with crash phases, of about 4 years' duration [Reference Ashby15].
An increased food supply may improve M. glareolus survival during the winter [Reference Mallorie and Flowerdew21] and lead to earlier onset of breeding the following year. In continental Europe, higher numbers of breeding adult voles lead to larger vole populations and an increased risk of virus transmission between voles [Reference Niklasson22], and from voles to humans. In years without large fluctuations, NE cases are often sporadic, with cases at a low level throughout the year with a slight increase in the summer months [Reference Groen23]. During ‘epidemic’ years, NE cases peak in spring or summer with a minor peak in early winter [Reference Clement24]. In Belgium, for example, numbers of human NE cases demonstrate a 3- and, more recently, a 2-year cyclicity (1990, 1993, 1996, 1999, 2001, 2003, 2005, 2007), with a striking connection to mast years [Reference Heyman12, Reference Clement14, Reference Clement, Maes and Van Ranst25]. After the very heavy mast year in 2004, a record number of NE cases (n=372) were reported in 2005 in Belgium, with notably large outbreaks in France and Germany [Reference Mailles7, Reference Hofmann9, Reference Koch11, Reference Clement14].
Comprehensive longitudinal datasets exist for beech mast years for GB. The occurrence of these mast years are associated with climate [Reference Matthews26], and are often synchronized with those in continental Europe [Reference Piovesan and Adams27, Reference Pucek28]. Heavy masting is thought to be stimulated by above-average temperatures and excess of sunshine, which favour flower bud formation, in July prior to masting [Reference Pucek28]. However, temperature records alone do not appear to correlate well with the occurrence of mast years [Reference Hilton and Packham29], and, for a number of countries, the incorporation of additional climatic variables appears to strengthen predictions [Reference Piovesan and Adams27]. For example, drought in the early summer a year prior to a mast year (Y−1) was highlighted as a very strong predictor in Europe, particularly when following an unusually moist, cool summer the year before (Y−2) [Reference Piovesan and Adams27]. However, frost in April of the mast year (Y0), appears to reduce seed production [Reference Matthews26, Reference Hilton and Packham29].
Certainly, there is evidence of HFRS disease in the British Isles, but the serotype involved in these case studies has very often been uncharacterized, so it remains uncertain which serotype, and indeed whether PUUV itself, was involved [Reference Walker30–Reference Pether and Lloyd32]. Some human serology studies have been carried out [Reference Lloyd and Morgan-Capner33, Reference McKenna34], again with the serotype not always reported; although seropositivity in one Scottish study was reported to be PUUV-specific [Reference Lloyd and Morgan-Capner33]. A serosurvey of cats in GB (using EIA and IIF testing) suggested that antibody to hantavirus (non-serotype specific) was widespread in cats, which, however, are not reservoirs for PUUV [Reference Bennett35]. Moreover, hantavirus is not a notifiable disease in the UK and there have been no large-scale human or animal studies on the potential for PUUV circulation.
This paper considers PUUV transmission in GB and investigates whether the British climate similarly impacts on GB mast years as it does in PUUV-endemic regions of northwest Europe. Mathematical models are applied to a range of GB climate variables over the period of an endogenous plant cycle [i.e. current (Y0) and the previous 2 years (Y−1, Y−2)], to assess their importance as predictors of mast years. Other possible ecological constraints on UK endemic transmission are discussed.
METHODS
Data on recorded British mast years (classified as ‘very good’ or ‘heavy’ masting) were obtained from various studies; primarily the Woodland Research Group, University of Wolverhampton (surveyed 100 trees over 16 years across England) and Forestry Commission reports (assessed 349 plots throughout Britain). These years were: 1976 [Reference Pucek28]; 1980, 1982, 1984, 1987, 1990 and 1995 [Reference Hilton and Packham29]; 1997, 2000, 2002, 2004 and 2006 [Reference Hendry, Boswell and Proudfoot36–Reference Redfern, Boswell and Proudfoot40]. Binary response variables [mast year (1), non-mast year (0)] were analysed with a number of explanatory variables that have previously been highlighted by other studies [April mean temperature in masting year (AT0), July mean temperature (JT−2, JT−1), and rainfall (JR−2, JR−1) 1 and 2 years respectively, prior to a potential masting event and July sunshine the year before masting (JS−1)] [Reference Matthews26–Reference Pucek28, Reference Redfern, Boswell and Proudfoot40, Reference Collinson and Sparks41] (Table 1).
Table 1. Regression of six single climate variables with masting events
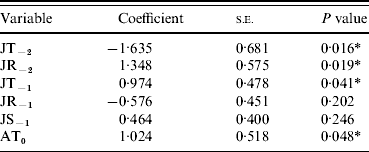
JT, July temperature; JR, July rainfall; JS, July sunshine; AT, April temperature.
Y−2=2 years before masting; Y−1=1 year before masting; Y0=year of masting.
* P<0·05.
Regression modelling
Analysis was performed using a Meteorological Office climate dataset for England and Wales (1976–2004) which includes data on monthly average temperature, rainfall and sunshine. Univariate and multivariate analyses were carried out to identify which combination of these variables (highlighted above) best predicted masting. The time series of each variable was analysed individually using binary logistic regression (Stata 7.0; StataCorp, USA), with masting as the response variable [mast year (1) or no mast year (0)], to determine their association (Table 1). Significant (P<0·05) variables from the univariate analysis were then entered into a multivariate logistic regression model and only variables that contributed significantly (P<0·05) to the multivariate model in a likelihood ratio test were retained (Table 2).
Table 2. Multivariate logistic regression model
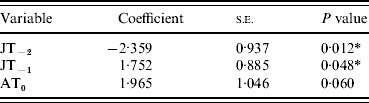
JT, July temperature; AT, April temperature.
Y−2=2 years before masting; Y−1=1 year before masting; Y0=year of masting.
* P<0·05.
In order to improve upon the multivariate model, a wider investigation was undertaken of the Meteorological Office data series. Thirty-month time-periods (February Y−2 to July Y0) were considered in order to capture the effects of climate on the endogenous plant cycle conditioning for masting or non-masting (e.g. bud formation, flower induction and pollination) [Reference Piovesan and Adams27]. All 93 climate variables (temperature, rainfall and sunshine for all months) with 29 samples (i.e. years) on each were analysed using a generalized linear model with binomial error distribution and a logistic link function, developed in R (R Project for Statistical Computing) [42]. Potential models were constructed by adding variables and testing for significant differences in fit between nested models, using the difference in residual deviance between models. The dispersion was estimated from the residual deviance and degrees of freedom of the larger model to form an F statistic with Bonferroni adjustment to the probabilities.
All possible one-, two- and three-variable models were tested for significant difference in fit from their parent models. However, due to overdispersion in the residuals, a quasi-binomial model was adopted to correct the significance estimates of the parameters. Those models for which the Fisher scoring algorithm failed to converge due to multiple co-linearity were ignored. Jack-knifing validation tests were performed on all models that exhibited significant differences in fit from their parent models, to determine predictive power and mean square predictive error (MSPE).
RESULTS
Regression modelling
As single predictors of masting events, only four of the variables showed significant (P<0·05) associations with mast years [April temperature in the mast year (AT0), July temperature 2 years before (JT−2), July rainfall 2 years before (JR−2), July temperature 1 year before masting (JT−1)] (Table 1). The only non-significant variables (JR−1 and JS−1), which were also highly correlated with JT−1, were therefore removed from the subsequent multivariate model. Of the four remaining variables, JT−2, JT−1 and AT0 contributed most significantly to the multivariate model. JR−2 did not contribute significantly and was removed (Table 2). AT0 was only marginally non-significant at the 95% level and so was retained. JT−2 was negatively associated with a masting event, whereas JT−1 and AT0 were positively associated.
Incorporation of 93 climate series variables (for temperature, rainfall and sunshine) into a generalized linear model showed that one- and two-variable models, when tested by jack-knifing, were not sufficiently reliable to predict masting. Considering three-variable models, 61 models exhibited a significant difference in fit to their parent models at the 95% level. When validated, through jack-knifing, resulting MSPE and the number of years correctly predicted (mast or non-mast years) were recorded. One model predicted 27 out of 29 years correctly and a further six predicted 26 years correctly. The eight models with a MSPE <0·05 and an ability to predict 27, 26 or 25 years correctly are presented in Table 3. The model with an MSPE <0·05 that predicted 27 years correctly was model 5 [a warm April in the mast year (AT0), high October sunshine 1 year before (OS−1) and high December sunshine 2 years before (DS−2); Fig. 1, Table 4]. The variable coefficients were all significant, it had a low MSPE score and few convergence errors. Encouragingly, two of the three variables in model 5, high April sunshine in the mast year (AS0) and high October sunshine the year before (OS−1), also appeared in five of the eight better-fitting models, suggesting their particular importance as predictors for a mast year. Exploration of four-variable models using the same method produced no models capable of predicting 27 years correctly.
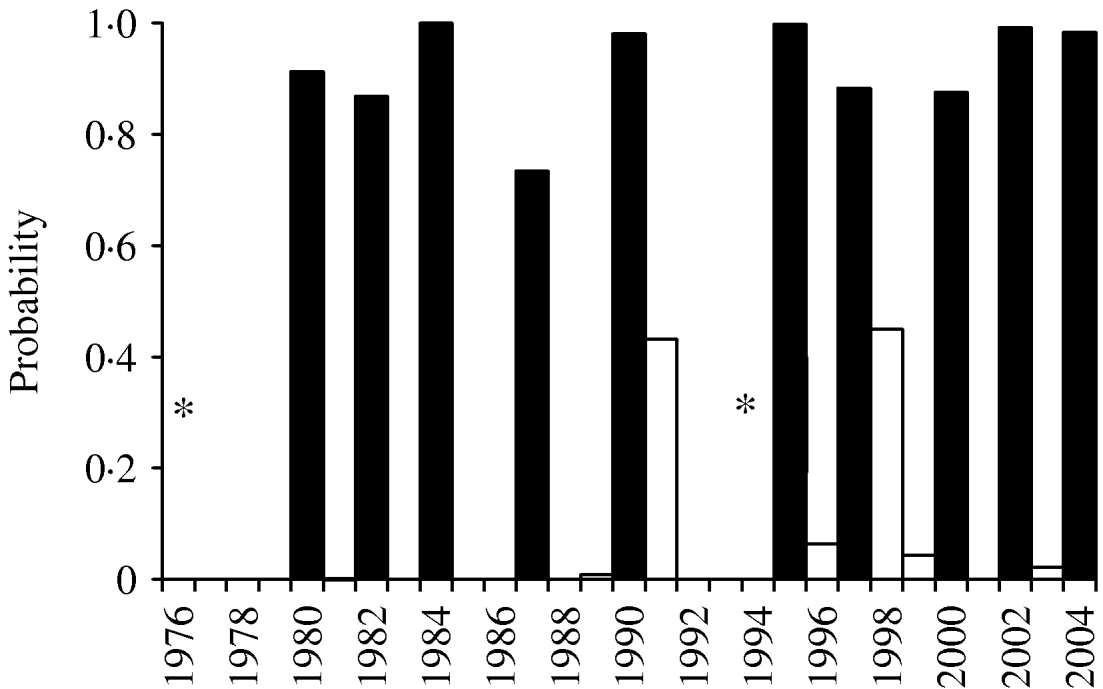
Fig. 1. Jack-knife validation fit for three-variable model 5 (April sunshine in the year of masting, October sunshine the year before masting and December sunshine 2 years before masting). * Model 5 failed to converge in 1976 (mast year) and 1994. ▪, Observed mast years; □, non-mast years.
Table 3. General linear model-derived three-variable models with highest mean square predictive error (MSPE)
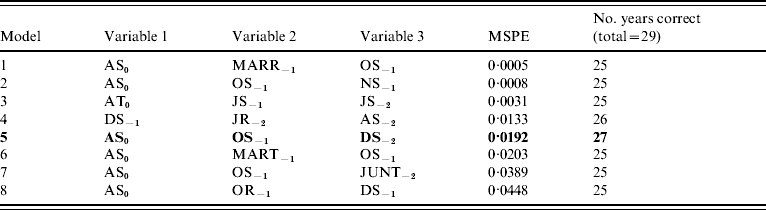
AS, April sunshine; AT, April temperature; DS, December sunshine; JUNT, June temperature; JR, July rainfall; JS, July sunshine; JT, July temperature; MARR, March rainfall; MART, March temperature; NS, November sunshine; OS, October sunshine.
Y−2=2 years before masting; Y−1=1 year before masting; Y0=year of masting.
Table 4. Model 5 output
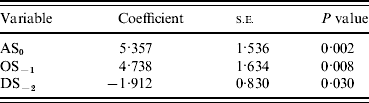
AS, April sunshine; OS, October sunshine; DS, December sunshine.
Y−2=2 years before masting; Y−1=1 year before masting; Y0=year of masting.
DISCUSSION
Our results confirm that good/heavy mast years in GB are significantly associated with a cold July 2 years before (JT−2), a hot July 1 year before (JT−1), and a warm April in the same year (AT0), as is the case in continental Europe. The more in-depth regression modelling applied to all potential climatic variables as predictors, identified one model (model 5) which could correctly predict 27 out of 29 years. However, this model did not highlight July climate as especially important, but did include high April sunshine (AS0) which is likely to be associated with a high AT0, a predictor previously found to be important for heavy masting. Likewise, high October sunshine the year before was also found to be a good predictor, and, interestingly, this combination appeared in five of the eight better-prediction models. These predictors (high April sunshine in the mast year and high October sunshine the year before) are likely to impact on beech trees in different ways. The former predictor permitting more flowers to survive in a milder spring thus allowing increased seed production, and the latter probably correlated with drought (see below). It is probable that certain extreme climatic factors [high October sunshine and high July temperature the year before masting (OS−1 and JT−1) and low July temperature 2 years before (JT−2)] increase stress on the adult tree which, as a survival strategy, promotes the retention of nutrients and production of flowers. An unusually dry autumn [e.g. high October sunshine the year before masting (OS−1)], may increase drought stress, thus promoting the retention of nutrients and encouraging greater crop production the following year [Reference Piovesan and Adams27].
Clearly, climate itself is not the only factor involved with beech tree masting patterns. Other suggested factors include nutrient supply, stand size and aspects of pollination as well as the inherent masting rhythm of the tree itself [Reference Hilton and Packham29]. Nevertheless, a combination of sequential climate variables in GB appears, in this analysis, to satisfactorily predict the occurrence of beech masting. Other authors have shown that these climatic variables are also linked to good/heavy beech masting in northwest Europe – where significant PUUV outbreaks have subsequently occurred [Reference Clement14, Reference Tersago43]. These variables could arguably be employed to predict both potential mast years and any risk of PUUV outbreaks in both continental northwest Europe and the UK.
Although these climatic factors are evidently important for masting, they may not be the only limiting or influencing factors involved in the ecology of PUUV transmission in GB. Other climatic factors, such as temperature and humidity, will influence the survival of M. glareolus (host ecology) as well as of the virus outside of the host (virus ecology) [Reference Linard44]. Good or heavy mast years usually prolong the breeding season and favour overwintering of voles, leading to increased numbers the next spring [Reference Ashby15–Reference Smyth17] and these numbers can fluctuate dramatically [Reference Southern and Lowe20]. However, other environmental factors, may also affect the ability of the host to overwinter. A cold winter and snow cover, for example, may influence mortality directly or indirectly through reduced predation [Reference Pucek28, Reference Hansson and Henttonen45] and a higher population density may increase mortality rates [Reference Jensen16]. Differences, such as habitat quality and spatial heterogeneity, may also play a part in asynchronous fluctuations in nearby M. glareolus populations which may only be separated by relatively short distances [Reference Alibhai and Gipps18]. A very good food supply, therefore, may be necessary, but not sufficient for winter breeding [Reference Smyth17]; however, there is no evidence so far that winter breeding has occurred without preceding good seed production [Reference Jensen16].
Since M. glareolus occurs ubiquitously in GB, this raises the question of why PUUV may not have been reported in voles or humans. The lack of significant evidence for NE could be attributed to limited disease awareness, with sporadic and/or isolated cases going unrecognized. This was probably the situation in Belgium and France, where only a low number of NE cases were registered before 1990, but where the disease is now endemic. Possibly this is because PUUV infections can have a pauci-symptomatic or asymptomatic clinical course, or cause relatively non-specific symptoms [Reference Clement, Maes and Van Ranst25, Reference Clement, Maes, Van Ranst and Tabor46]. However, even despite the fact that there is no specific hantavirus surveillance in GB, it seems unlikely that any major NE outbreaks, such as those that occur in continental Europe, would go unnoticed.
There are other possible mitigating reasons as to why Britain could be ecologically less suitable for PUUV transmission. The stands of beech woodland common to Britain are much smaller and more fragmented than those that occur in continental Europe. The reduced impact that beech masting, in smaller more fragmented woodlands, would have on voles might lead to isolated, mast-influenced populations, leading to a discontinuous distribution of potentially infected populations that do not mix [Reference Linard44, Reference Tersago47]. It has been hypothesized that the risk of human infection results from an increase in the mass shedding of virus, after a population increase of infected voles over a threshold density. Therefore, compared to continental Europe, these discontinuous populations may limit the possibility for reaching a required critical population threshold density necessary for transmission cycles of PUUV to be maintained. Indeed, the highest levels of PUUV infection in European M. glareolus were almost invariably found at the highest density peaks of these same populations [Reference Olsson4, Reference Escutenaire5, Reference Niklasson22, Reference Clement24, Reference Clement48], although others have found evidence of higher hantavirus incidence in more fragmented landscapes. However, this was related to Sin Nombre virus in deer mice in the USA [Reference Langlois49]. If there is a critical threshold that is not met, then there might be no, or limited, spillover of virus between voles, and therefore no, or reduced, exposure to humans [Reference Escutenaire5, Reference Linard44, Reference Tersago47]. In such a scenario, risk areas would likely be localized, with any cases being sporadic. Rates of contact between voles and humans, and therefore risk of infection, will probably also differ with human behaviour patterns and this exposure is arguably due in part to the degree of woodland fragmentation, as well as to weather conditions [Reference Clement14, Reference Clement24]. Recreational and occupational activities which might increase human exposure to infected vole droppings – such as hunting, camping or staying in forest shelters/lodges – are probably associated with more continuous woodland such as that of endemic PUUV areas in continental Europe (and Fennoscandinavia) but less common in the fragmented woods of Great Britain. Finally, because the British bank vole population is more isolated than that on the continent, it could be that PUUV infection in voles, should it have occurred here, may have suffered extinction due to the stochastic nature of infection [Reference Keeling and Rohani50]. However, these hypotheses need to be explored further and the results, where appropriate, incorporated into future, more sophisticated models. The ecology of PUUV is evidently complex, as is the impact of weather on beech masting. Recent reports have suggested that climate change will have significant effects on beech in the UK, with increased drought leading to a greater frequency of beech masting [Reference Broadmeadow, Ray and Samuel51]. This is likely to impact significantly on vole ecology and PUUV epidemics.
Evidently, the clinical significance of PUUV in GB remains to be determined. Clarification, however, would require active surveillance; such as routinely adding hantavirus serology to investigations of suspected leptospirosis cases – as others have previously encouraged [Reference Clement, Maes and Van Ranst25, Reference Kudesia31, Reference Clement, Maes, Van Ranst and Tabor46, Reference Davies52]. This paper argues that the host ecology and the UK environment could indeed support the presence of PUUV, and provides a means to understand how and when beech masting is influenced by climate and its effect upon PUUV host populations. Learning from the current PUUV outbreaks and research in continental Europe and applying it appropriately to GB, provides a useful means to target surveillance of the virus in both M. glareolus populations and suspected human cases and may present an important component in the development of predictive tools for zoonotic diseases.
ACKNOWLEDGEMENTS
This forms part of the Health Protection Agency (HPA)'s work on emerging zoonoses, and is funded through HPA Government Grant-in-Aid. The HPA is an executive agency of the Department of Health. The views expressed are those of the authors and not necessarily those of the HPA or the Department of Health.
DECLARATION OF INTEREST
None.







