INTRODUCTION
Blastomycosis is a rare, but potentially fatal infection caused by the thermally dimorphic fungus Blastomyces dermatitidis. The disease is prevalent in parts of North, America, Africa and India [Reference Smith and Kauffman1]. The disease primarily affects humans and dogs who are predominantly, but not exclusively, exposed to rural agricultural, recreational or wilderness environments [Reference Smith and Kauffman1–Reference Morris3].
Establishing the diagnosis of blastomycosis is often challenging. In 39–54% of cases, the infection is asymptomatic [Reference Smith and Kauffman1, Reference Klein4]. In patients who are symptomatic, illness typically begins 30–45 days after exposure and often manifests as mild self-limited respiratory infection [Reference Smith and Kauffman1, Reference Klein4]. Therefore, in many cases the disease does not come to the attention of a physician and/or has a high risk of being misdiagnosed. Hence, awareness of the endemic regions for blastomycosis is critical to diagnosing this infection.
In Canada, the disease is endemic to provinces that surround the Great Lakes (i.e. Ontario, Manitoba) [Reference Dwight2, Reference Morris3, Reference Crampton5, Reference Kepron6]. The disease has also been reported in Alberta, Nova Scotia, Saskatchewan and New Brunswick [Reference Crampton5, 7–Reference Ross9]. In the past, case reports and small case series suggested that B. dermatitidis may be endemic in the province of Quebec [Reference Sekhon, Bogorus and Sims7, 10–Reference Grandbois13]. However, to date no study has established the incidence rate of blastomycosis in the province. Therefore, it remains unknown whether Quebec represents an important endemic region for blastomycosis in North America.
In the current work we review 158 cases of human blastomycosis that were diagnosed in the province of Quebec during 1988–2011, establish the incidence rate and define the geographical distribution for this infection in the province.
METHODS
In Quebec, all cases of blastomycosis that are identified by all local and tertiary-care hospitals are sent for confirmation to the provincial public health laboratory in Sainte-Anne-de-Bellevue or to a mycology laboratory at the Hôtel-Dieu de Québec Hospital, Laval University, which serves as a satellite for the provincial public health laboratory in Quebec City. The provincial health plan does not cover validation tests that are performed outside the province; therefore, analysis of records from these two laboratories is likely to yield a good estimate of incidence for culture and histopathologically proven blastomycosis in the province. The records for microbiologically confirmed cases of blastomycosis have been maintained since 1988.
In June 2011, de-identified data on all human blastomycosis cases documented since 1988 was provided by the aforementioned laboratories. For each case the data included patient's age, sex, residence by administrative region (county), date of diagnosis, organ site for each specimen and method used to establish the diagnosis (i.e. culture vs. histopathology). These results were analysed using a linear regression model by XL-STAT software (Addinsoft Inc., USA). Census population and annual population estimates were used to calculate annual incidence rates [14].
RESULTS
Our case finding revealed 158 cases of human blastomycosis that were diagnosed in Quebec between January 1988 and June 2011. According to our findings, 75·3% (119 cases) of individuals affected were men, while only 24·7% (39 cases) were women. The age distribution of cases spanned individuals from 1 to 94 years with the highest incidence recorded in 40–74 years age groups (Fig. 1a). Organs, from which microbiological samples were obtained, included lungs (94 cases), skin (33 cases), bones (six cases), joints (four cases) and genitourinary tract (three cases). In 18 cases the organ site was not known and for six (4%) of cases the diagnosis was established using histopathological findings.

Fig. 1. Review of 158 cases of blastomycosis in Quebec documented during 1998–2011. (a) Number of diagnosed cases of blastomycosis in various age groups in the province. (b) Linear regression model demonstrating the occurrence of blastomycosis in Quebec (R 2=0·418, P=0·001). The slope of a trend line was calculated as 0·37 cases/year. (c) Linear regression model analysis of annual incidence of blastomycosis per 100 000 people in Quebec (R 2=0·369, P=0·002). The slope of a trend line was calculated to be 0·0044 cases/100 000 individuals per year.
Overall, the annual number of documented cases in the province has increased over the past 20 years (Fig. 1b). Specifically, an average of 10·3±3·6 cases of blastomycosis were diagnosed in Quebec during 2005–2010, which is more than double the number of cases diagnosed during 1988–1998 (4·4±3·2). Based on linear regression model analysis the observed increase is statistically significant (P=0·001). Using annual population estimates [14], the annual incidence rate of blastomycosis per 100 000 individuals was calculated and analysed using the linear regression model (Fig. 1c). This graph demonstrates that the incidence of blastomycosis also steadily increased over the last 20 years (P=0·002). Based on 2005–2010 results this annual rate was documented as 0·133 cases/100 000 individuals.
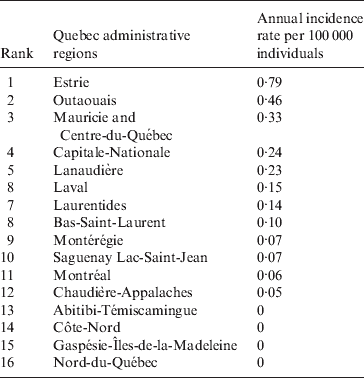
Cases of blastomycosis were documented in all but four administrative regions (counties) of Quebec during 2005–2010 (Fig. 2, Table 1). Most cases of blastomycosis were reported from the southern part of the province. No cases of blastomycosis were documented during 2005–2010 in the northern regions of the Côte-Nord, Nord-du-Québec, Abitibi-Témiscamingue and Gaspésie, although these areas are less densely populated. The highest annual incidence rates for blastomycosis, based on results for 2005–2010, were recorded in Estrie (0·79 cases/100 000 population), Outaouais (0·46 cases/100 000 population), Mauricie and Centre-du-Québec (0·33 cases/100 000 population) and Capitale-Nationale (0·24 cases/100 000 population) administrative regions (Fig. 2).

Fig. 2. Annual incidence rate of blastomycosis per 100 000 people reported for each administrative region in Quebec during 2005–2010.
Table 1. Annual incidence rate of blastomycosis per 100 000 people reported for each administrative region in Quebec during 2005–2010
DISCUSSION AND CONCLUSION
Our results reveal that ∼10·3±3·6 cases of human blastomycosis are diagnosed in Quebec each year, which equates to an annual incidence of ∼0·133 cases/100 000 individuals, based on 2005–2010 data. The occurrence of blastomycosis based on the number of cases diagnosed and population-adjusted incidence rate has been steadily increasing over the past 20 years. The observed rise in occurrence of blastomycosis may be due to technical advances in medicine and our increased ability to establish a diagnosis or due to increased migration from urban to more rural areas, and an increase in popularity of camping, hiking and other wilderness activities.
Quebec covers a significant territory; therefore, it is important to consider regional distribution for this infection. Geographically, the disease was limited to the southern part of the province with the highest incidence in regions bordering New Hampshire (Estrie administrative region) and Ottawa, Ontario (Outaouais administrative region) (annual incidence rates of 0·79 and 0·46 cases/100 000 population, respectively). In comparison, these regional incidence rates are significantly lower than the annual incidence in Kenora, Ontario (7·11 cases/100 000 population) [Reference Dwight2, Reference Morris3]. However, these rates are comparable to other known endemic regions such as Manitoba or Mississippi (0·62 and 1·3 cases/100 000 individuals, respectively) [Reference Crampton5, Reference Chapman15].
Our study has a number of limitations. As stated earlier, blastomycosis is asymptomatic in 39–54% of cases and a significant number of cases are mild or self-limiting. Furthermore, it is possible that some physicians treated patients empirically for this infection, without sending a tissue or sputum sample for confirmation. In addition, in Quebec, fatal pneumonia, which could be secondary to B. dermatitidis, often does not trigger autopsy and microbiological confirmation and, therefore, would be missed by the provincial public health laboratory. Thus, the method used in this study to identify blastomycosis patients probably underestimates the actual clinical disease burden in the province. Moreover, while our study documented a doubling in the occurrence of blastomycosis in the province over the last 20 years, since the collected data did not include detailed patient information, we were not able to comment on factors that might have precipitated this increase.
In summary, this study confirms Quebec as an endemic area for B. dermatitidis. Physicians should be aware to its presence as a potential pathogen in residents and visitors to this region.
ACKNOWLEDGEMENTS
This work was supported by a Canadian Dermatology Foundation research grant to Dr Litvinov.
DECLARATION OF INTEREST
None.





