INTRODUCTION
Shigella bacteria are enteropathogens that cause diarrhoea and bacillary dysentery, and humans are their principal reservoir. Endemic and epidemic Shigella strains are a common cause of infection and even mortality in the developing countries, whereas in Western countries most of the infections are caused by imported strains [Reference Ekdahl and Andersson1]. S. sonnei (serogroup D, a single serotype), which causes a relatively mild disease, most commonly infects travellers from Western countries [Reference Ekdahl and Andersson1–Reference Janda and Abbott5]. S. flexneri (serogroup B, consisting of six serotypes with several subtypes) is endemic in most developing countries and causes more mortality than the other Shigella spp. S. dysenteriae (serogroup A, consisting of 15 serotypes) causes large epidemics in many developing countries. The fourth species, common in some parts of the world, is S. boydii (serogroup C, consisting of 20 serotypes). Three predominant strains are globally responsible for the majority of shigellosis cases, namely S. sonnei, S. flexneri 2a and S. dysenteriae type 1 [Reference Sur6].
Although gastroenteritis caused by Shigella spp. is normally self-limited, patients are often treated with antimicrobial agents to reduce the duration of the illness and the period of shigella excretion after symptoms subside [Reference Heymann7]. However, Shigella strains have rapidly developed resistance to the commonly used antimicrobials [Reference Niyogi8]. Ampicillin and trimethoprim–sulfamethoxazole previously used to treat shigellosis are of limited use today. Nalidixic acid was commonly used in the early 1990s but has since lost its effectiveness in many regions. Fluoroquinolones, especially ciprofloxacin, are the drug of choice in Western countries today. However, Shigella strains seem to become rapidly resistant to this group of antimicrobials as well. Here we report on the increasing antimicrobial resistance among Shigella spp. isolated from patients in Finland between 1990 and 2005.
METHODS
Shigella strains
All of about 30 Finnish routine clinical microbiology laboratories have sent, first voluntarily and since 1995 mandatorily, all their suspected Shigella isolates to the National Reference Centre for Shigella at the Enteric Bacteria Laboratory of the National Public Health Institute (KTL). The potential history of travel preceding the diarrhoea of a patient was supplied on a special form that always accompanied the strain.
This study was based on 1814 isolates that were obtained during 1990–2005. Standard identification methods were used. Serotypes were determined by using commercially available antisera (Wellcome Diagnostics, Dartford, UK, and since 1995 Denka Seiken, Tokyo, Japan) according to the manufacturer's instructions. If the results of the biochemical identification or of the serotyping were doubtful, the strain was referred to the Laboratory of Enteric Pathogens of the Health Protection Agency (Colindale, London, UK) for verification.
The origin of an isolate was defined in our study as a continent where the infected person had travelled prior to falling ill. The whole of Russia or Former Soviet Union was considered as part of Europe. The American continents were considered as one group since only 10 of the 158 American isolates were from North America. In the course of the study Asia was divided into two: the Far East including China, India and the neighbouring countries (Pakistan, Bangladesh, Nepal, Sri Lanka) and the rest of Asia: other Asian countries including Turkey.
Susceptibility testing
During 1990–1998, antimicrobial susceptibility of Shigella isolates to the following 12 antimicrobials was determined: ampicillin, chloramphenicol, streptomycin, sulfonamide, tetracycline, trimethoprim, ciprofloxacin, nalidixic acid, mecillinam, imipenem, neomycin and ceftriaxone. During 1999–2005, gentamicin and cefotaxime were used instead of neomycin and ceftriaxone. The susceptibility tests were performed by agar diffusion technique, first, on Iso-Sensitest medium using the zone size criteria recommended by the disc manufacturer (A/S Rosco, Taastrup, Denmark) and established by the Swedish Reference Group for Antibiotics [9]. CLSI guidelines [10] and discs from Oxoid (Oxoid, Basingstoke, Hampshire, UK) were first used in 1999 and Mueller–Hinton agar in 2005. In this study, the strains showing intermediate susceptibility were categorized as resistant. Since 2000, minimal inhibitory concentration (MIC) for ciprofloxacin has been determined by E-test (AB Biodisk, Solna, Sweden) to the isolates resistant to nalidixic acid, following the recommended MIC breakpoints, susceptible⩽1 mg/l and resistant⩾4 mg/l [10]. MIC of 0·125–1·0 mg/l was considered as reduced susceptibility to ciprofloxacin [Reference Cheasty, Day and Threlfall2].
Statistical methods
χ2 test (Epi-Info, v. 3.3.2, CDC, Atlanta, GA, USA) was used to evaluate the significance of the increased number of antimicrobial-resistant strains. When an expected cell value was <5, Fisher's exact test was used. Statistical significance was indicated at P<0·05. The proportion of multi-resistant Shigella strains in different areas of the world was modelled using binomial distributions Bin(N(t), p(t)), where N(t) is the total amount of strains in the year t. The probability (proportion of multi-resistant strains) p(t) was modelled using log-link as log (p(t))=a+b∗(t−1990), where parameters a and b must be estimated. The goodness-of-fit tests were used to assess the fit of the linear time trend in log-scale. The relative annual change in the proportion that was multi-resistant was exp (b)−1.
RESULTS
In Finland, around 100 cases of shigellosis have been reported annually during the last decade. Of the 1869 isolates originally available for the study, the following were excluded: 41 isolates from an outbreak caused by contaminated foodstuff in 2001, nine isolates from an outbreak in a shelter for alcoholics in 1990 and five isolates from a hospital outbreak in 1991. All these outbreaks were caused by S. sonnei. Thus, 1814 strains (one isolate per patient) were analysed. Of these, 68% (1238 strains) belonged to S. sonnei, 22% (407 strains) to S. flexneri, 7% (123 strains) to S. boydii and 3% (46 strains) to S. dysenteriae.
Of the 1814 Shigella strains analysed, 1592 (88%) were known to be associated with travel abroad: 37% originated from Asia (13% from the Far East and 24% from the other Asian countries), 29% from Africa (most commonly Egypt), 13% from Europe (especially Russia and Estonia), 9% from Latin America and 0·5% from the United States (Table 1). Further, 10% of the patients had no trips outside Finland 4 weeks before the symptoms started and for 2% no suspected location of infection was indicated. While S. sonnei was the most common Shigella isolate detected and seen relatively equally in travellers returning from all locations, all other Shigella spp. were most common in travellers returning from Africa (Table 1). There were no major annual changes in the proportions of different species at each geographic area during 1990–2005 (data not shown).
Table 1. Number of Shigella strains associated with travelling to different geographic areas during 1990–2005
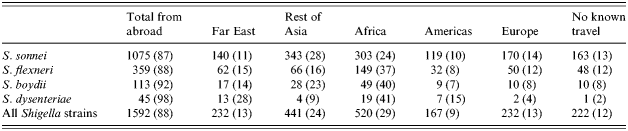
The proportion of strains by species originating from various geographical areas is shown in parentheses (%).
During the study period, the proportion of multi-resistant shigellas rose globally (Tables 2 and 3). A binomial model from the data indicated that the overall proportion of resistant strains increased significantly (P<0·005), which was mainly due to the increasing proportion of resistant strains imported from Asia (often from Turkey or Thailand, P<0·001) (Table 2, Fig. 1). The strains originating from the Far East were usually already multi-resistant at the beginning of the study period, yet the proportion continued to increase (P<0·001) (Table 2, Fig. 1).

Fig. 1. Predicted proportion of multiresistant strains in the Far East (- - -), the rest of Asia (—) and Africa (– – –).
Table 2. Number of the isolates from subjects with known history of travel (n=1592) to certain geographical areas outside Finland and the annual proportion of multi-resistant (resistant to ⩾4 antimicrobials) strains (%) in 1990–2005

The proportion of resistant strains to all the antimicrobial agents studied increased during the study period. Statistically significant changes in the sensitivity of the strains can be seen with several antimicrobials between the first two and last two years of follow-up (Table 3). However, the frequencies of resistance differed between the species (Table 3). For example, 19% of the S. sonnei strains but 12% of the S. flexneri strains were nalidixic acid-resistant. In addition, the proportion of multi-resistant (resistant to ⩾4 antimicrobials) S. sonnei strains nearly doubled in 15 years, whereas for the other species the change was not statistically significant. The number of S. boydii and S. dysenteriae isolates was too small to draw any firm conclusions.
Table 3. Comparison of antimicrobial resistance (%) of 702 Shigella strains isolated during two periods (in 1990–1991 and 2004–2005). Multi-resistant strains were resistant to ⩾4 antimicrobial agents and pan-susceptible strains were susceptible to all tested agents

Significance of the change in the proportion of resistant strains during the indicated times, measured by χ2 test: *** P<0·001, ** P<0·01, * P<0·05; n.s., not significant; —, not tested; n.d., not determined.
Isolates from all the countries were commonly resistant to several ‘traditional’ antimicrobials (ampicillin, chloramphenicol, streptomycin, sulfonamide, tetracycline and trimethoprim). However, most of the strains from the Far East have now developed resistance to one or more of the following antimicrobials: mecillinam, nalidixic acid, ciprofloxacin, gentamicin and cefotaxime. During the years 1990–1997, nalidixic acid resistance of the isolates from travellers returning from the Far East remained low (between 0% and 6%) but in 1998 resistance started to increase so that in 2003 six strains (67%), in 2004 14 strains (61%) and in 2005 20 strains (87%) originating from the Far East were resistant to nalidixic acid (data not shown). In general, only one strain resistant to nalidixic acid per year has been obtained from the other countries.
Resistance to nalidixic acid indicates possible lowered resistance to fluoroquinolones. During 2000–2005, a total of 65 nalidixic acid-resistant strains were found and 86% of them originated from Far East countries. In 2000–2001 susceptibility to ciprofloxacin had decreased (MIC ⩾0·125 mg/l) in 44%, in 2002–2003 in 62% and in 2004–2005 in 83% of the nalidixic acid-resistant strains. The first isolate highly resistant to ciprofloxacin (MIC 32 mg/l) was encountered in 2004 and the second (MIC 6 mg/l) in 2005. The ciprofloxacin resistance of these two plus one other strain (MIC 3 mg/l) was also detected by the disc method. All these isolates belonged to the S. flexneri 2a serotype. During 2004–2005 the rest of the S. flexneri strains (n=9) also showed reduced susceptibility to ciprofloxacin, whereas 23% of the S. sonnei strains (n=26) were still completely susceptible to ciprofloxacin (MIC <0·125 mg/ml).
We have tested resistance to gentamicin since 1999, and during 2004–2005 five gentamicin-resistant strains were detected. Two strains belonged to S. flexneri (from China) and three to S. sonnei (two from China and one from Egypt). In addition, in 2002 we received a S. sonnei strain originating from Egypt that was resistant to cefotaxime and to five other antimicrobials (ampicillin, streptomycin, sulfonamide, tetracycline and trimethoprim). Several mecillinam-resistant Shigella strains have been seen since 1990. Thus, resistance to representatives of nearly all groups of antimicrobial agents was detected in Shigella. Of the 12 antimicrobials tested, only resistance to imipenem was not detected during the study period.
DISCUSSION
In this study we used the so-called ‘epidemiological susceptibility panel’ of 12 different antimicrobial discs for Shigella antimicrobial susceptibility testing. A similar panel is also frequently used in other countries belonging to the Enter-net international surveillance network for human gastrointestinal infections. The antimicrobial panel showed that the proportion of multi-resistant Shigella strains is growing and, moreover, resistance to several new antimicrobials is emerging. Development of resistance to some clinically important antimicrobials in Shigella isolates from Finns has been followed since 1975. The current data, together with this previous study [Reference Heikkilä11], showed that, for example, the development of resistance to trimethoprim increased from 0% to nearly 80% in 20 years (Fig. 2). So far, the resistance to nalidixic acid has also risen from 0% to nearly 20% in 10 years. However, the resistance to nalidixic acid is much more common in the Far East than in the other areas of the world. Similarly, ciprofloxacin resistance is now increasing in the Far East, especially among S. flexneri. As a consequence, Finnish travellers who have been to those areas can now be infected with a ciprofloxacin-resistant strain.

Fig. 2. Development of resistance to some clinically important antimicrobial agents in Finnish Shigella isolates during 1975–1988 [Reference Heikkilä11] and 1990–2005 (this study). Sul, sulfonamide; Tmp, trimethoprim; Tet, tetracycline; Amp, ampicillin; Mec, mecillinam; Nal, nalidixic acid; Cip, ciprofloxacin.
Ciprofloxacin is currently the recommended antimicrobial for the treatment of shigellosis worldwide [Reference Heymann7, 12], and also in Finland. Although several studies have established the tolerability and efficacy of fluoroquinolones in children during short-term treatments [Reference Phavichitr and Catto-Smith13, Reference Leibovitz14], the use of cephalosporins has been recommended for children instead of ciprofloxacin [Reference Cheasty, Day and Threlfall2]. Thus far we have detected only one strain resistant to a third-generation cephalosporin. However, it is of concern when considering the alternatives for treatment with ciprofloxacin. It can be expected that Shigella strains will rapidly develop resistance to cephalosporins as well, since the emergence of extended-spectrum of β-lactamases have been reported in recent years, for example, from Korea in S. sonnei isolates [Reference Kim15], from Argentina in S. flexneri isolates [Reference Andres16] and from Bangladesh in S. sonnei, S. flexneri and S. boydii [Reference Rahman17].
The Finnish Shigella isolates commonly originate from the countries popular among Finnish tourists, especially Egypt, Turkey, India, Tunisia and Estonia. The antimicrobial resistance patterns detected among these isolates reflect the resistance situation and usage of antimicrobial agents in the countries the infections were obtained from. Each year we also receive Shigella isolates from Finns who have not travelled abroad before contracting shigellosis. The antibiotic resistance patterns of these strains are similar to those of foreign origin; these people may have received their infection as a secondary infection or from imported foodstuffs. The total number of detected cases of shigellosis has decreased during the study years; the exact reasons for this are unknown. However, increased hygiene standards at holiday resorts and increased awareness of personal hygiene have possibly led to this positive development.
South and East Asia seem to be the areas where the new resistances emerge. In some areas of China, virtually all Shigella strains are resistant to nalidixic acid [Reference Wang18, Reference von Seidlein19]. Moreover, a high prevalence of resistance to ciprofloxacin has been reported in China, in one study 6% [Reference von Seidlein19] and in another as much as 20% [Reference Wang20]. Resistance to gentamicin was also reported to be rather common (13%), since oral gentamicin is widely used to treat bacillary dysentery in China, even if its therapeutic efficacy is controversial [Reference Wang20]. In China and in parts of India, S. flexneri 2a is the dominant Shigella type [Reference Wang20, Reference Pazhani21]. In Calcutta, in 2004, 92% of the S. flexneri strains were resistant to nalidixic acid and 41% to ciprofloxacin, in contrast to the S. sonnei strains, which were all resistant to nalidixic acid but none to ciprofloxacin [Reference Pazhani21]. Similarly, in Bangladesh, during 1999–2003, more than 60% of S. sonnei isolates were resistant to nalidixic acid but none to ciprofloxacin or other newer antimicrobials [Reference Talukder22]. However, ciprofloxacin-resistant S. sonnei strains have recently been reported from Japan [Reference Ahmed23]. The difference in the resistance patterns of S. sonnei and S. flexneri was also reflected in the strains Finnish travellers were infected with.
We found that isolates originating from the Americas, Europe or Africa were often multi-resistant but rarely exhibited resistance to nalidixic acid or reduced susceptibility to ciprofloxacin. This agrees with previous reports on the situation in these areas [Reference Streit24–Reference Ahmed28]. However, in the Middle East and parts of Africa the incidence of resistance seems to be increasing [Reference Ashkenazi29, Reference Eja30]. Studies conducted in other Western countries, where the majority of shigellosis cases are related to travel abroad, have also reported on a growing prevalence of multi-resistant strains and, especially, an increase in nalidixic acid resistance [Reference Cheasty, Day and Threlfall2–Reference Hirose4]. In England and Wales, of the 912 Shigella strains isolated in 2002, 13% of S. sonnei and 10% of the other strains were resistant to nalidixic acid. All these strains were reported to have reduced susceptibility to ciprofloxacin (MIC 0·125–1·0 mg/l), although no clinically significant resistance (MIC ⩾2 mg/l) was detected [Reference Cheasty, Day and Threlfall2].
Since virtually all the Shigella isolates from the Finnish travellers were available to our study, we were able to analyse where the Finns became infected with shigellosis and what the resistance patterns of these isolates were. Naturally, our study does not give a full picture of the situation in the various destination countries. This could only be provided by an improved surveillance system in each of these countries. However, in our study, certain differences in the antimicrobial resistance were clearly seen. First, the possibility of ciprofloxacin resistance has to be taken into consideration when treating patients from Asia, especially from China or India. Second, S. flexneri strains are more probably ciprofloxacin resistant than the strains of the other species. This knowledge offers a basis for empiric treatment recommendations. However, our study also showed that the antimicrobial resistance situation is changing, thus, in the future, constant surveillance will be needed in Finland as well as in other countries.
ACKNOWLEDGEMENTS
We thank Jukka Ollgren, M.Sc., from the Departments of Infectious Disease Epidemiology and Vaccines for generating the binomial model.
DECLARATION OF INTEREST
None.







