INTRODUCTION
In early 1909 a young and unknown Brazilian physician-cum-protozoologist, Carlos Ribeiro Justiniano das Chagas (1879–1934), was sent to investigate an outbreak of malaria that was disrupting the construction of an important railway in the state of Minas Gerais [Reference Scliar1]. There he came across a new and strange disease, known to the locals as baticum (pronounced ‘bah-tee-coom’) [Reference Scliar1], an onomatopoeic word for the palpitation resulting from the heart condition characteristic of the illness – American trypanosomiasis or Chagas' disease. The condition, first confounded with syphilis, caused cardiac insufficiency and a significant number of sudden deaths.
Chagas was pondering the causes of that strange disease and how it was transmitted when one of the railway engineers, drew his attention to the large number of triatomine bugs in the area. These were known locally as barbeiro (barber) due to their habit of biting humans on the face [Reference Scliar1]. When Chagas examined the intestines of the bugs he found the trypomastigote forms of trypanosomes, the causal agent of American trypanosomiasis. Since Chagas was familiar with the characteristics of vector-borne tropical diseases such as malaria and yellow fever, the detection of a hitherto unknown trypanosome in the gut of a reduviid bug prompted him to search for a related vector-borne disease [Reference Perleth2]. A few weeks after the discovery of the trypanosome in the bug he examined a baby girl, who had presented with a strange swelling of one eyelid, fever and malaise [Reference Punukollu, Gowda and Khan3]. A drop of her blood revealed the same trypanosomes found in the bugs – the cycle was completed.
With this first human case identified, Chagas completed an extraordinary cycle of work, without precedence in the history of medicine thus far: he discovered a new disease, identified its agent and its transmission mechanism within the space of a few weeks. Chagas was twice formally nominated for the Nobel Prize in 1913 and 1921 but was never awarded it.
Less than 100 years later Brazil became the first Latin American country to eliminate transmission of Chagas' disease by Triatoma infestans, its main vector in the country [Reference Punukollu, Gowda and Khan3]. The Pan American Health Organization (PAHO) marked the milestone on 9 June 2006 by presenting the Minister of Health with an International Elimination of Transmission of Chagas' Disease Certificate. The achievement was confirmed by an international expert commission based on visits to every Brazilian state [4, 5].
In this review I have addressed the elimination of Chagas' disease transmission. Following a summary of the infection, emphasizing its transmission mechanism and its distribution in Brazil, I have discussed its main determinant of transmission. I then analysed the possible determinants of its elimination, the strategy used, the difficulties encountered, lessons learned, the future for world eradication of Chagas' disease, and what it means to the populations no longer affected.
I have also calculated the basic reproduction number, R 0, for Chagas' disease and demonstrated that its relatively low value (1·25) explains why vectorial transmission was interrupted fairly easily. In addition, I have used a mathematical model to forecast how long the remaining cases of the disease, as well as the additional vertically transmitted cases will last.
THE DISEASE
American trypanosomiasis (Chagas' disease) is a zoonosis caused by the protozoan parasite Trypanosoma cruzi (a homage to Oswaldo Cruz, the head of Carlos Chagas' department at the time of the discovery) [Reference Kirchhoff, Guerrant, Walker and Weller6]. The disease has two phases: acute and chronic.
Acute Chagas' disease [Reference Rassi, Rassi, Rassi, Brener, Andrade and Barral-Neto7] is usually an illness of children, but it can occur at any age [Reference Kirchhoff, Mandell, Bennet and Dolin8]. The acute phase begins after an infected bug bites a susceptible human and defecates in the spot [Reference Rey9, Reference Beaty and Marquardt10]. Some hours later, the biting site produces a characteristic swelling called ‘chagoma’ [Reference Marsden and Cook11]. Unilateral orbital oedema, lymphangitis and satellite ganglia engorgement, the so-called Romaña sign, should always suggest acute Chagas' disease in an endemic area [Reference Marsden and Cook11]. Fever and tachycardia develop. An ECG may show alterations in ventricular repolarization, subepicardic ischaemia and first-degree atrioventricular block [Reference Marsden and Cook11]. Acute phase infection is not usually fatal and often passes unperceived. After a few weeks the disease settles into the more chronic phase. The clinical manifestations of chronic Chagas' disease, however, appear decades after the acute phase. Cardiomyopathy, the commonest manifestation, is characterized by extrasystoles (hence the name baticum) and various degrees of atrioventricular block. Valvular incompetence is also a common feature and is caused by the characteristic cardiac dilatation, which sometimes evolves to apical aneurysm [Reference Marsden and Cook11]. Sudden death due to ventricular fibrillation [Reference Prata, Lopes and Chapadeiro12] and as a result of autonomic cardiac dysfunction [Reference Junqueira13] is frequently recorded.
Other chronic manifestations of Chagas' disease are the so-called megasyndromes [Reference Marsden and Cook11], in particular megaoesophagus and megacolon [Reference Resende, Brener, Andrade and Barral-Neto14]. The former is characterized by a progressive difficulty in swallowing and the latter by chronic constipation.
Congenital transmission may occur at any time of pregnancy, in successive gestations and may affect twins. The infection may produce pathology in the growing foetus. The consequences for the newborn are variable, ranging from asymptomatic to severe clinical manifestations. Congenital transmission cannot be prevented, but early diagnosis of the newborn enables prompt treatment, achieving cure rates close to 100% (the treatment regimen should include benznidazol between 5 and 10 mg/kg per day for 30–60 days or nifurtimox at 10–15 mg/kg per day for 60 days), thus avoiding progression to chronic Chagas' disease. It is a consensus that congenital Chagas' disease will be a pressing public health concern until the pool of infected women of childbearing age decreases to insignificant levels, which may only happen 30 years hence.
Transmission
The principal mechanism of Chagas' disease transmission is by the bite of insect vectors called triatomine bugs [15]. These blood-sucking bugs become infected by biting an infected animal or person. The vector belongs to the subfamily Triatominae (Hemiptera: Reduviidae) [Reference Schofield16–Reference Schofield18] comprising 130 recognized species, of which about a dozen can transmit the trypanosome.
The bugs are found in houses made from materials such as mud, adobe, straw, and palm thatch. During the day, the bugs hide in crevices in the walls and roofs. During the night, when the inhabitants are sleeping, the bugs emerge. Because they tend to feed on people's faces, triatomine bugs are also known as ‘kissing bugs’. Infected bugs pass T. cruzi in their faeces.
After they bite and ingest blood, they defecate. The person becomes infected if T. cruzi parasites in the bug faeces enter the body through mucous membranes or breaks in the skin. The unsuspecting, sleeping person may accidentally scratch or rub the faeces into the bite wound, eyes, or mouth.
Other forms of transmission include: consumption of uncooked food contaminated with faeces from infected bugs; congenital transmission (from a pregnant woman to her baby); blood transfusion; organ transplantation; and accidental laboratory exposure [15].
Chagas' disease is not transmitted from person-to-person.
Chagas' disease distribution in Brazil before the intervention programme
The disease, which probably had its origins in Brazil, is limited to the Western Hemisphere. Most reported cases occurred in Brazil, Argentina, Chile and Venezuela [Reference Allison and Kiple19]. Cases have also been reported in Peru, Mexico and other Central and South American countries.
Trypanosoma cruzi, the causal agent of Chagas' disease, is found exclusively in the Americas. It is a member of the class Mastigophora, family Trypanisomidae and has over 100 vertebrate hosts, including dogs, cats armadillos, opossums, monkeys, and humans [Reference Allison and Kiple19]. It is present in all habitats of its triatomine vectors, between latitudes 41o N and 46o S [Reference Rey9, Reference Moncayo20]. It is estimated that, in the 1980s, about 80 million people lived in areas with risk of transmission [Reference Kropf, Azevedo and Ferreira21, 22].
The endemic area in Brazil, not surprisingly, coincides with the geographical distribution of Triatoma infestans, its main vector (Fig. 1), and comprises 2 million km2, a quarter of the national area. In 1980 there were 120 000 new cases. In the period between 1975 and 1978 the Ministry of Health of Brazil carried out a seroprevalence survey in the endemic area and found 4·1% individuals positive, equivalent to about 800 000 cases of the disease [Reference Rey9].

Fig. 1. Geographical distribution of Triatoma infestans in South America (from [Reference Schofield, Jannin and Salvatella23]).
Of historical interest is the case of Charles Darwin, who wrote that he was bitten by a huge Triatoma while in South America [Reference Allison and Kiple19]. According to Burnet [Reference Burnet24] Darwin's mysterious chronic illness, from which he suffered, dated from this time, and he cited a distinguished protozoologist who believed that Darwin was infected with Chagas' disease.
Chagas' disease as a vector-borne infection
Between 1877 and 1910 eleven different infections including Chagas' disease were shown to require a blood-sucking arthropod vector for transmission to humans [Reference Philip, Rozenboom, Smith, Mitler and Smith25, Reference Gubler26]. Most of the techniques used for control and eradication of those vector-borne diseases were developed in the early 20th century. Reduction of vectors' breeding places, insecticides, biological control, vaccination, chemotherapy and personal protection were established nearly a century ago [Reference Beaty and Marquardt10]. Many of those techniques are still effective; others succeeded initially but failed to achieve eradication later for a variety of reasons. Investigators must now incorporate new approaches that will allow them to move to the next level of control to alleviate the effects of vector-borne diseases on human and animal health [Reference Beaty and Marquardt10].
The rest of this section is crucial for explaining why Chagas' disease transmission was eliminated from Brazil. The equations given are important for understanding the causes of the interruption of transmission.
The central parameter related to the intensity of transmission of infections is the so-called basic reproduction number (R 0), defined by Macdonald [Reference Macdonald27, Reference Dietz28] as the number of secondary human infections produced by a single infective in an entirely susceptible population. Originally applied in the context of malaria, R 0 is a function of the vector population density as related to the host population, m, the average daily biting rate of the vector, a, the host susceptibility, b, the vector susceptibility, c, the vector mortality rate, μ, the parasite extrinsic incubation period in days, n, and the parasitaemia recovery rate, r, resulting in the famous equation:
where exp (−μn) is the fraction of the infected vector population that survives through the extrinsic incubation period n of the parasite.
From the definition of the basic reproduction number it is clear that if R 0 is <1, the disease dies out. Hence, in the original Macdonald analysis, R 0 coincides with the threshold for infection persistence, i.e. R 0=1.
In his seminal paper, Macdonald [Reference Macdonald27] addressed the problem of a system involving one vector (Anopheles mosquitoes) and one host (man). As mentioned above, his definition of R 0 is the number of secondary infections produced by a single infected person along one entire infectious period. I shall deduce an explicit expression for R 0 from an intuitive perspective to show that it coincides with the threshold for the establishment of the disease.
Let us begin by assuming that the index case is a human host. The question to be answered is how many human secondary infections this index case produces in one entire infectious period.
Let N m be the number of arthropod vectors and a the average daily biting rate vectors inflict on the human population. The number of bites in the human population per day is, therefore, N ma. Let N h be the number of humans and r the rate of recovery from parasitaemia in the human cases. Therefore, the index case produces
infected vectors, where c h→m is the probability that a vector becomes infected after biting an infective human. Those (N ma/N hr)c h→m infected vectors, in turn, produce
new human cases in the first generation, where (l/μ) is the average life expectancy of mosquitoes, b m→h is the probability that a human gets the infection after being bitten by an infective mosquito and exp (−μn) is the fraction of the infected vector population that survives through the extrinsic incubation period n of the parasite. Note that once infective, a vector is assumed to remain so for life. Therefore, the expression for R 0 is [Reference Lopez29]:
which is just another way of writing equation (1).
A simple sensitivity analysis shows to which of its components R 0 is most sensitive [Reference Burattini30–Reference Marques, Forattini and Massad35]. This can be done by taking the partial derivative of equation (4) with respect to each of the parameters. Each of these partial derivatives represents one control strategy. So, for instance, to estimate the impact of bed nets on the value of R 0 we take the derivative with respect to the biting rate, a, because bed nets reduce the contact between the vector and the human host. The result is:
Another way to reduce the contact between vectors and human hosts is the use of repellents in infected people. This would, in a sense, protect the vector (like bed nets) and, therefore, the partial derivative would be with respect to the product ac, i.e.
Improving housing conditions would reduce the breeding places for Triatoma vectors and this, in turn, would reduce the number of adult vectors N m and the partial derivative should be with respect to this variable:
Treatment of infected people would increase the recovery rate from parasitaemia, r. Hence we have:
Finally, the use of insecticides has the effect of increasing the vector mortality rate, i.e. the parameter μ. The sensitivity equation is now:
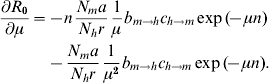
Numerically, the parameter to which R 0 is most sensitive is the vector mortality rate.
Another important aspect of R 0 is its relationship with the prevalence level of human disease at equilibrium [Reference Molineaux, Wernsdorfer and McGregor36], P:
Considering that the prevalence of Chagas' disease at equilibrium before the beginning of the intervention programme in the endemic regions was 16 million infected people in an 80 million population at risk [37] we can calculate the value of R 0 in this area, which is 1·25.
Let us now assume a control strategy of spraying insecticides regularly in the affected areas. This strategy leads to an increase in the vectors' mortality rates. The impact of such a strategy can be simulated, beginning at the equilibrium value of R 0 estimated as 1·25 for the affected areas in Brazil and the result can be seen in Figure 2.

Fig. 2. Impact of a control strategy on the value of R 0.
Note that an increase of only 25% in the basal mortality rate of the vector is enough to reduce the value of R 0 below the critical threshold of 1, thus making the disease disappear from this area. As discussed below this is the main explanation for the success of the elimination programme against vectorial transmission of Chagas' disease.
The control strategies
The strategy to control Chagas' disease consisted essentially of interrupting transmission by domestic vector control and ensuring safer blood transfusion.
Chagas recognized the importance of improving living conditions to control the vector [Reference Dias, Silveira and Schofield38]. He noticed that bugs lived in cracks and crevices in the house walls, made by bamboo sticks and mud (Fig. 3) and realized that improved buildings would reduce the breeding and hiding places for the bugs.

Fig. 3. Triatoma infestans and its life cycle in the wall of a typical house in the endemic area. (1) Eggs, (2) first-stage nymphs, (3) fifth-stage nymph, (4) adult (from [Reference Geigy and Herbig39]).
Since improving housing conditions is an expensive strategy and was applied only in restricted cases [Reference Silveira40] insecticide spraying became the most widely used control measure. The efficacy of systematic fogging with insecticides since the first developments of gamma-hexachlorocyclohexane (BHC) in 1947 [Reference Busvine and Barnes41] recommended its use as the first control alternative [Reference Silveira40]. The possibility of controlling domestic triatomine vectors with a variant of that new insecticide was demonstrated in the following year [Reference Dias and Pellegrino42]. This triggered the first National Campaign against Chagas' disease in 1950 [Reference Silveira40] by the National Service of Malaria. For many years BHC remained the mainstay of Chagas' disease vector control trials and campaigns, although dieldrin was widely used in other countries, e.g. Venezuela [Reference Dias, Silveira and Schofield38]. Until the advent of synthetic pyrethroid insecticides in the early 1980s, several classes of insecticides were trialled against triatomine vectors, but none showed the efficacy and copst-effectiveness of BHC and dieldrin.
In 1983 the first Brazilian national campaign was launched and by 1986 75% of the initial objectives had been attained, in the sense that infested localities had been mapped, sprayed, and placed under community-based surveillance [Reference Dias, Silveira and Schofield38]. Unfortunately, by that epoch dengue re-emerged in Brazil and the almost complete mobilization of the Brazilian public health systems towards dengue control placed the Chagas' disease campaign subordinate to a new urban Aedes aegypti campaign.
In 1991, the Southern Cone Initiative, a joint agreement between the governments of Argentina, Bolivia, Brazil, Chile, Paraguay, Uruguay and Peru, to control Chagas' disease by the elimination of the main vector, Triatoma infestans was signed. This initiative has been highly successful and the prevalence of the vector plummeted in the last years [Reference Schmunis, Brener, Andrade and Barral-Neto43].
This programme consisted of three operational phases [Reference Moncayo20]:
(1) preparatory phase for mapping and general programming of activities and estimation of resources;
(2) attack phase during which a first massive insecticide spraying of houses followed by a second spraying 2–3 months later, with additional evaluations for selective spraying of re-infected houses; and
(3) surveillance phase for the detection of residual foci of triatomines after the objective of the attack phase has been reached.
The primary objective of the Southern Cone Initiative was the elimination of T. infestans, including suppressing or controlling populations of other species that might be of local importance. The second objective was to reduce the risk of Chagas' disease transmission by blood transfusion. Transfusional Chagas' disease was suspected in early 1940 and definitively defined in the 1940s [Reference Dias, Silveira and Schofield38], although the tools to control it were only developed 10 years later [Reference Dias, Silveira and Schofield38, Reference Wendell, Dias and Coura44]. However, it was only with the emergence of HIV/AIDS in 1980s that national programmes of blood control were fully implemented [Reference Wendell, Dias and Coura44–Reference Massad46]. The strategy to control transfusional Chagas' disease consisted in blood screening by serological test and chemoprophylaxis of suspected blood with trypanocidal drugs [37, Reference Wendell, Dias and Coura44].
Vertical transmission, the third most important way of acquiring Chagas' disease, was known since the 1940s [Reference Dias, Silveira and Schofield38]. Although the estimated probability of transplacental transmission of T. cruzi varies considerably, it may reach 10% [37, Reference Bittencourt47]. Control has been restricted to early diagnosis and specific treatment of infected newborns [Reference Dias, Silveira and Schofield38].
Difficulties encountered
The first difficulty encountered with controlling Chagas' disease by attacking the vector was the cost of the proposal to improve housing conditions. At the time of Chagas' discovery in 1909, Brazil had about 24 million inhabitants [Reference Mortara48], of whom 5 million were at risk of acquiring the infection [assuming R 0≅1·25; see equation (9)]. This implied improving about 1 million dwellings. The cost of restoring each house to control triatomine breeding conditions was estimated at between US$500 and US$1000 in present-day US$, making a total cost between US$0.5 and US$1 billion. Considering that the gross national product (GNP) of Brazil at that time was US$7.2 billion [Reference Reis49] (in 1999 US$), between 7% and 14% of the GNP would be needed to control the infection by improving housing conditions. This was an unattainable task.
The second difficulty concerned the use of synthetic insecticides, developed in 1940s, for vector control. DDT, the cheapest and widely applied insecticide, was ineffective against the main vector Triatoma infestans [Reference Dias, Silveira and Schofield38]. Two new organochlorines were developed, dieldrin and BHC, which were shown to be very effective when sprayed over house walls [Reference Romaña and Abalos50]. As previously mentioned, BHC was replaced in the 1980s by synthetic pyrethroid insecticides. These were considerably more expensive than BHC, but because they could be used in low doses, their ease of use, infrequent application and lack of unpleasant smell [Reference Dias, Silveira and Schofield38], they proved to be cost-effective and advantageous. House spraying with synthetic pyrethroid became the standard strategy against Chagas' disease. The main difficulty, however, faced by this strategy was the re-emergence of dengue in 1986. This vector-borne infection drew all the attention of the Brazilian Public Health authorities at that time. Diversion of resources toward A. aegypti is still common up to present days. About 90% of the budget of the Centre of Zoonosis Control (CZC) was directed to dengue control, the remaining 10% for rabies control.
Further difficulty was that the resources applied in the vectorial control of Chagas' disease were left over from the malaria control programme, malaria having been eradicated from triatomine-endemic areas [Reference Vinhaes and Dias51]. This fact compromised the planning of control actions that should be strictly based on epidemiological criteria based on information derived from entomological and serological screenings carried out at the time.
All these above difficulties were eventually overcome and vector control has remained the main strategy against the disease. The combination of a relatively low value of R 0 associated with persistent house spraying with insecticides proved to be highly effective against triatomine vectors and the result is the certification of transmission elimination Brazil managed to obtain.
Pre-transfusional serodiagnosis, the main strategy to control transfusional Chagas' disease also presented some difficulties. The first problem was the lack of legal instruments and regulations that could enforce donor selection. The regulations date from the 1980s [Reference Dias and Schofield45]. As late as 1995, only three of the six South Cone Initiative countries had a specific law controlling blood donation [52]. In addition, operational difficulties related to the first screening test applied (complement fixation) were only overcome with the advent of new techniques like immunofluorescence, indirect haemmaglutination and ELISA [53]. However, perhaps the greatest difficulty in controlling transfusional Chagas' disease was the huge migration flow of infected individuals from endemic areas to the great urban centres since the 1950s [Reference Souza, Ramirez, Bordin, Dias and Coura54]. This resulted in the fact that in the 1970s, of the 100 000 new cases of Chagas' disease per year in Brazil, 20 000 were due to transfusion, of which 1500 new cases occurred in the city of São Paulo, a non-endemic area [Reference Dias, Brener and Andrade55]. As mentioned earlier, the emergence of HIV/AIDS improved the quality of blood screenings and transfusional transmission of T. cruzi ceased to be an important way of acquiring the infection in Brazil.
Congenital transmission of T. cruzi infection was also targeted. The success in the control of vector-transmitted Chagas' disease and screening programmes in blood banks uncovered the public health relevance of congenital transmission, which has been gradually emerging in vector-free suburban areas and non-endemic cities [Reference Schijman56]. The main difficulty in controlling it was due to the lack of an efficient pre-natal programme that could diagnose the infection in candidate mothers coupled with a safe chemoprophylaxis to then reduce the likelihood of vertical transmission. This is the greatest challenge in eradicating Chagas' disease from Brazil since there still remain an estimated 8000–16000 new cases per year of congenital transmission [Reference Schijman56]. Although congenital transmission cannot be prevented, early diagnosis enables prompt treatment of newborns achieving cure rates close to 100% [Reference Schijman56].
The impact of Chagas' disease control
In Brazil the control programme has been operating since 1975. At that time 711 of the >5000 municipalities had triatomine-infested houses targeted by the programme [Reference Moncayo20]. In 1986, 186 cities remained infested and in 1993, 83 municipalities infested with triatomines were detected. The current geographical distribution of T. infestans in South America (Fig. 4) shows that, compared with the area displayed in Figure 1 of 6 278 081 km2 [Reference Gorla57], the current estimate is 913 485 km2 [Reference Schofield, Jannin and Salvatella23]. This illustrates the impact of control strategies. Note that Brazil is free from T. infestans.

Fig. 4. Geographical distribution of Triatoma infestans in South America as a result of the control programme (from [Reference Schofield, Jannin and Salvatella23]).
The effectiveness of the South Cone Initiative is measured using various parameters. The estimated burden of disease in terms of disability-adjusted life years (DALY) [Reference Murray and Lopez58] declined from 2·7 million in 1990 [Reference World59] to 586 000 in 2001 [Reference Mathers, Lopez, Murray, Lopez, Mathers, Ezzati, Jamison and Murray60]. From 1975 to 1995, the programme (excluding blood banks) prevented an estimated 89% of potential disease transmission, preventing 2 339 000 new infections and 337 000 deaths in the whole region [Reference Moncayo20].
Reports from Brazil in the late 1980s suggested that the aggregate cost for pacemakers and intestinal surgery for Chagas' disease was US$250 million per year. This excludes the costs of consultations, care, and supportive treatment for chronic chagasic patients, which amounted to US$1000 per year per patient, and disability awards, which in one state accounted for US$399 600 [Reference Dias61, Reference Schofield and Dias62]. In contrast, the annual investment cost was estimated as US$300 000 [Reference Schofield, Jannin and Salvatella23]. In addition, cost-effectiveness analysis demonstrated that for each US$39 spent on the programme 1 DALY was gained.
The significant reductions in the percentage of houses infested with triatomines were accompanied by a rapid reduction in the frequency of acute cases [Reference Dias63, Reference Souza64]. In addition, vector control was also associated with important reduction in transmission by secondary mechanisms such as transfusional and congenital transmission. Therefore, the prevalence of the infection in the 0–4 years age group was reduced from 5% in 1980 [Reference Camargo65] to 0·12% in 2000 [66], an impact of 98%.
Another important aspect of transmission interruption is the impact on the clinical features of chronic infections. There was a significant reduction in morbidity and premature mortality, suggesting an indirect benefit due to the absence of re-infection. Previous studies in the 1960s [Reference Dias67] and 1970s [Reference Macedo68] already suggested that a reduction of re-infection, due to reductions in vector infestations, might be responsible for the declining morbidity in chronically infected individuals.
As previously mentioned, following the emergence of HIV/AIDS, strict control of blood banks helped to dramatically reduce the number of transfusional cases of Chagas' disease. Almost all Latin American endemic countries now have legislation regulating the screening of blood for transfusion [Reference Moraes-Souza69]. This control, linked to vector control, is contributing to a progressive reduction in the number of people acquiring the infection by blood transfusion [Reference Wendell, Dias and Coura44].
Finally, serological screening of children born to chagasic mothers 6 months after birth (serology performed before 6 months could reflect transplacental antibodies), with immediate treatment of those positive is reducing the number of vertically transmitted cases of Chagas' disease. This strategy, associated with vector control, is expected to have an important impact in reducing congenital transmission because the important reduction in re-infection reduces the likelihood of transmission to women of childbearing age [Reference Dias, Silveira and Schofield38], and there are preliminary indications suggestive of a decline in the vertical transmission due to this lack of re-infection [Reference Dias, Coura, Dias and Coura70].
The future
Presently there are about 3·5 million people living with Chagas' disease in Brazil [Reference Kropf, Azevedo and Ferreira21]. It would be very convenient to have an estimate of the time when the disease can be considered as eradicated, i.e. when there is no individual living with the disease. For this, it is necessary to project the current number of individuals considering age-dependent prevalence and mortality rates. By applying simple demographic models we can estimate how long it will take for the disease to disappear from the country, considering the current status of no vector and transfusional transmission and the residual congenital transmission.
If we assume the estimated age distribution of Chagas' disease prevalence [71] and mortality rate [72] we can calculate, with demographic models [Reference Smith and Keyfitz73], the half-life of each age group. The result can be seen in Figure 5.

Fig. 5. Age distribution of half-life of number of cases in each age group.
It can be seen in the figure that the 15–29 years age group, for instance, has a half-life of 30 years. This implies that, from the current 480 000 estimated cases in this age group, 240 000 will still be alive in 30 years time. Moreover, from the current 227 500 estimated cases for the 0–4 years age group, in 40 years there will be 113 750 individuals alive, and so on. Therefore, even if the transmission were completely interrupted now, it would take several decades before complete eradication of cases.
It is possible, with the use of a simple mathematical model (described in the Appendix) to forecast [Reference Massad74] the total number of Chagas' disease cases from vertical transmission. Therefore, assuming that from the currently estimated 2·5 million cases of Chagas' disease, 51% or 1·785 million are women. Assuming also the estimated age distribution of Chagas' disease prevalence [71] and mortality rate [72], and the age distribution of female fertility [75] and general mortality rate for Brazil [76], we can estimate the time evolution of cases of congenital Chagas' disease. The result of the simulation of the model described in the Appendix, assuming different proportions of treated children can be seen in Figure 6.

Fig. 6. Forecast of Chagas' disease assuming infected mothers and congenitally infected children for the next 50 years. The proportion of treated children is 0% (upper curve), 20%, 40%, 60% and 80% (lowest curve).
Note that, beginning with the currently estimated number of infected women in the reproductive age of 760 000 cases, there is a marked reduction in the total number of cases (the sum of infected mothers and children), which is not very sensitive to treatment. Note also that the number of cases will linger for several decades.
CONCLUSIONS
The successful elimination of vectorial and transfusional transmission of Chagas' disease is a result of the South Cone Initiative. Authorities managed to reduce domestic density of the primary vector T. infestans and to achieve almost 100% of coverage in blood serological selection. As previously mentioned, the basic reproductive number of Chagas' disease was close to 1, i.e. not far from the threshold. Therefore, an average reduction of 25% in the vector life expectancy (feasible thanks to the domestic habits of T. infestans) was enough to reduce R 0 below unity, and to achieve the elimination of this form of transmission. However, control cannot be relaxed. Other secondary vectors, like T. brasiliensis in Northeast Brazil show peridomestic habits and new methods to control such peridomestic populations are still needed.
From the five requirements for the existing programmes summarized in the literature [Reference Schofield and Dias77–Reference Vinhaes79], the first two: (1) to maintain the political priority of the programme until its consolidation (5–10 years), and (2) to improve and refine epidemiological surveillance, that must become more focused at peripheral administrative levels supported by regional and national technical reference groups, are practically fully attained in Brazil. There remain the other three, namely, (3) to improve and refine methods and strategies for the control of peridomestic infestation by secondary vector species; (4) to cover 100% of blood transfusion with prior serological selections; and (5) to improve medical and social attention to the remaining chagasic individuals'. Of these, the achievement of items (4) and (5) are well advanced and the main attention should be focused on the control of secondary vectors. In particular in the Amazon region, we have a worrying number of new cases of Chagas' disease, as well as evidence of progressive domestication of vector species such as Panstrongylus geniculatus, Triatoma maculata and Rhodnius brethesi [Reference Coura80].
But perhaps the greatest challenge to avoid recrudescence of Chagas' disease transmission in Brazil is the so-called ‘curse of success’ [Reference Schofield, Jannin and Salvatella23], whereby success in reducing the epidemiological burden invariably also reduces political interest and operational budgets [Reference Schofield, Jannin and Salvatella23]. The need for continued surveillance and intervention becomes less appreciated at the political level [Reference Dias, Silveira and Schofield38] and there is a dangerous tendency for political authorities to underestimate the potential resurgence of vectorial transmission and give priority to emergent infections, as occurred with dengue in the early 1980s. It should be borne in mind that although the reproductive number of Chagas' disease is currently below unity, there are more than 100 mammal species that harbour the parasite and negligence from the health authorities would be sufficient for it to return to its pre-control level. That would be a tragedy.
For an interesting historical account of R 0 see [Reference Dietz28] and for a detailed account of R 0 for complex diseases see [Reference Lopez29–Reference Marques, Forattini and Massad35].
APPENDIX
This Appendix describes the dynamical model used for forecasting the total number of Chagas' disease cases for the next decades.
The model assumes a population divided into four states, namely, infected mothers, M, uninfected newborns, F, infected newborns, C, and treated and recovered children, R. The dynamics of the model is explained by the following set of equations:
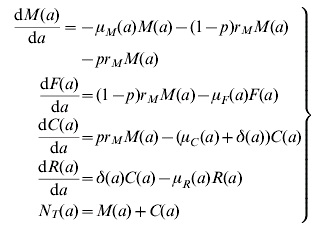
where a stands for age and the definition of the parameter and their values are detailed in Table A1.
Table A1. Parameter definitions and values used in the model (A1)

* The Heaviside θ-function [Reference El'sgol'ts81] (a step function that is equal to 0 when the argument is <0, and 1 when the argument is ⩾0) was used in order to guarantee that women reproduced at a rate of 2% per year up to age 49, ceasing to do so from then onwards.
ACKNOWLEDGEMENTS
Thanks are due to Professor Francisco A. B. Coutinho for comments, criticisms and very stimulating discussion on the original manuscript, to Professor Norman Noah for the detailed editing and comments and also to an anonymous reviewer who pointed to several mistakes in the original version of the manuscript.
DECLARATION OF INTEREST
None.









