INTRODUCTION
Legionnaires' disease (LD) is a potentially fatal form of pneumonia primarily caused by inhalation of water aerosols containing Legionella. In the USA, about 25% of LD cases are travel associated [Reference Benin, Benson and Besser1, 2]. LD outbreaks among travellers have been described in association with large hotels [Reference Fraser3, Reference Benin4], cruise ships [Reference Rowbotham5–Reference Guerrero and Filippone7], and whirlpool spas [Reference Benkel8–Reference Vogt10]. The incubation period of LD is typically 2–10 days, but can be ⩾2 weeks [Reference Fraser3, Reference Den Boer11]. Consequently, infected travellers often disperse from the area where exposure occurred before developing symptoms, making it unlikely that any two patients sharing a travel-associated exposure will be seen by the same clinician or reported within the same public health jurisdiction. In 2005, state and local health departments and the Centers for Disease Control and Prevention (CDC) began enhanced surveillance for travel-associated legionellosis to detect travel-associated clusters nationally and prevent disease through remediation of identified environmental sources [Reference Benin4, 12].
Following reports from different states of three LD cases in persons who had travelled to a Las Vegas time-share condominium complex immediately before their onset of illness in 2001, CDC assisted the Southern Nevada Health District (SNHD) with an investigation. In 2008, CDC's travel-associated legionellosis surveillance system detected another cluster of four cases of LD in travellers visiting the same complex between October 2007 and September 2008. We conducted epidemiological and environmental investigations to identify the contamination source, prevent transmission, and determine whether the 2008 outbreak resulted from colonization of potable water with the same L. pneumophila sequence type that caused the outbreak in 2001.
METHODS
Epidemiological investigation, 2001
For case-finding, probable LD cases were defined as persons hospitalized with radiographically confirmed pneumonia who had stayed overnight at the condominium complex between 1 January 2001 and 10 August 2001 and became ill between 2 days after arriving and 14 days after leaving. Confirmed cases met the above criteria, with or without hospitalization, and had laboratory evidence of Legionella infection, including isolation of Legionella from respiratory secretions or demonstration of Legionella pneumophila serogroup 1 (Lp1) antigen in urine [13]. In 2001, complex managers mailed letters to the >16 000 guests who had registered between 1 January 2001 and 10 August 2001. Persons who developed pneumonia within 2 weeks of their departure were asked to contact CDC by telephone. Requests for reports of LD cases in Las Vegas residents or visitors were also sent to all physicians in Clark County, Nevada, state health departments, and the European Working Group for Legionella Infections (www.ewgli.org).
We conducted a matched case-control study in 2001. For each confirmed or probable case, we attempted to enrol four randomly selected controls from condominium guests; controls were matched based on arrival date. Because cases appeared to be clustered in one of the three towers of the complex (‘tower 2’), two sets of controls were obtained: guests who stayed anywhere in the complex and guests who stayed in tower 2. One adult guest residing in the registered guest's household was randomly selected for enrolment. This random selection was accomplished by requesting a list of the adult members of the current household who had stayed with the guest during the designated dates; a random number table and predetermined rules were used to select one person for interview. We used a standardized questionnaire to collect information on exposure to water sources, illness during or subsequent to visiting the complex, and presence or absence of underlying conditions associated with increased risk of LD (diabetes mellitus, cancer, emphysema, cystic fibrosis, bronchitis, asthma, immunological deficiency, organ/bone transplant, and heart, kidney or liver disease) [Reference Marston, Lipman and Breiman14]. Informed consent was obtained.
Matched odds ratios (mORs) and 95% confidence intervals (CIs) were calculated to assess univariate and multivariable relationships between categorical exposure variables and disease. Case-control sets with similar attributes were pooled to form larger matched sets [Reference Kleinbaum15]. Using conditional logistic regression, each model contained a single environmental exposure along with patient-specific characteristics that were potentially associated (P<0·1) with LD in the univariate analysis. In the model, age was categorized as <65 years or ⩾65 years. Statistical interactions were assessed between all possible pairs of variables. Collinearity diagnostics were performed using condition indexes. For all statistical tests, an alpha level of 0·05 was used to determine statistical significance; all P values reported are two-sided.
Environmental investigation, 2001–2002
An environmental investigation was initiated in August, 2001. The condominium complex is composed of a 19-storey central tower (tower 2) and two 18-storey adjacent towers (towers 1 and 3), joined in the shape of an ‘H’ (Fig. 1). To identify the environmental source of contamination, we documented the design of the potable water system and identified other potential sources of aerosolized water. Facility managers and engineers provided details of the water management system (e.g. halogen-based disinfection of water).
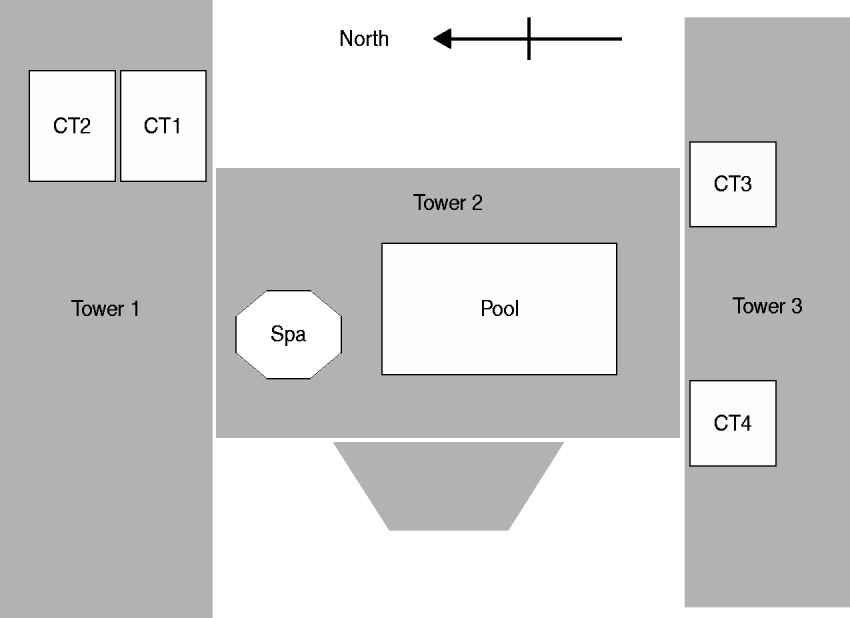
Fig. 1. Aerial view of tower structures and rooftop cooling towers (CT), spa, and swimming pool at a condominium complex in Las Vegas, Nevada, 2001.
According to established procedures [16], we collected biofilm swabs and 1-litre bulk water samples from the evaporative cooling towers, swimming pool, whirlpool spa, cabana misters, lobby fountain, and water heaters (n=23 samples). Moreover, we collected hot-water samples from sink taps and showerheads in three guest rooms in tower 2, where guests who developed LD had stayed and where unaffected guests had stayed (n=17). First, the inside of sink taps and showerheads were swabbed. Next, we allowed running water to heat before collecting bulk samples, so that the hot-water supply could be sampled adequately. Samples were collected from distal sections of potable water system loops throughout the complex. In addition, we measured water temperatures and bromine or chlorine concentrations [in milligrams per litre (mg/l)] in the potable water system as well as the cooling towers, swimming pool, whirlpool spa, and municipal water supply. Condominium complex maintenance records were also reviewed.
In 2002, SNHD personnel who had been involved in the initial environmental investigation in 2001 collected additional samples using the same sampling methods as the initial investigation. Swabs and bulk samples were collected from bathroom and kitchen sink taps and the bathtub in a room in tower 2 where a newly identified guest who developed LD had stayed; samples from water heaters serving tower 2 were also collected. All environmental specimens were shipped to the CDC Legionella laboratory.
At the CDC laboratory, bulk water samples were filtered through polycarbonate 0·2 μm filters. Filters were placed in 5 ml sterile water and vortexed for 60 s. Next, 100-μl aliquots of this suspension were placed on buffered charcoal yeast extract (BCYE) media with and without antibiotics. Plates were incubated at 35–37°C in 2·5% CO2 and read at 4 and 7 days. As standard practice, four Legionella colony-forming units (c.f.u.) per culture plate were selected for isolation and identification. Legionella species and serogroup were identified by slide agglutination using absorbed rabbit antiserum.
Epidemiological investigation, 2008
In 2008, we queried CDC's travel-associated LD surveillance database for cases in travellers to the complex between 2001 and 2008. Other case-finding strategies were similar to the 2001 methods, except that guests who visited the condominium complex between 1 August 2008 and 15 October 2008 and guests arriving between 16 October 2008 and 30 December 2008 received a letter from SNHD instructing those with onset of symptoms consistent with LD within 10 days following a condominium stay to seek medical attention and show the letter to a physician, who would contact SNHD to report the potential LD case. Guests who had stayed at the condominium and had received a diagnosis of pneumonia were instructed to contact SNHD by telephone. A case definition similar to the 2001 definition was used, but receipt of antimicrobial treatment effective against Legionella replaced hospitalization as a criterion for probable cases. An updated version of the same questionnaire was used to interview patients for a 2007–2008 case series.
Environmental investigation, 2008
The methods for collection of environmental samples were similar in 2008 and 2001 [16]. In October, we collected samples from the evaporative cooling towers, swimming pool, whirlpool spa, lobby fountain, and water heaters (n=30 samples). Cabana misters were no longer operational. Because colonization of potable water in tower 2 was suspected, we sampled four guest rooms near or exactly where guests who developed LD had stayed (n=22). We also sampled two randomly selected rooms in towers 1 and 3, where cases among guests had not occurred (n=18), and two municipal water feeds (n=2). Molecular sequence-based typing (SBT) was performed to create seven-gene, allelelic profiles (flaA, pilE, asd, mip, mompS, proA, neuA) and determine sequence types (ST) of select Lp1 isolates [Reference Gaia17, Reference Ratzow18]. SBT results from the 2008 environmental isolates and banked isolates from the 2001–2002 investigation, including environmental isolates and a clinical isolate obtained from an endotracheal aspirate, were compared.
Remediation and monitoring, 2001–2008
We reviewed available maintenance and service records related to the potable water supply from January 2002 to October 2008. The review included reports of the ongoing, environmental assessments performed by SNHD and records from facility managers and the industrial hygiene firm that was contracted to monitor for Legionella through environmental sampling and oversee operation of the chlorine dioxide system used for potable water disinfection.
Human subjects
This study was determined to be an emergency public health investigation exempt from institutional review board approval.
RESULTS
From 2001 to 2008, a total of 16 confirmed and 19 probable LD cases were identified in travellers to the time-share condominium complex (Fig. 2). No cases were detected in non-US travellers or condominium employees. In 2001, we identified two other confirmed cases and 16 probable cases in residents of 13 states (in addition to the three confirmed cases that prompted the investigation). One fatality occurred in a probable case (a 62-year-old woman with lymphoma). The five confirmed cases were infected with Lp1. Four cases were diagnosed by urinary antigen testing; one was diagnosed by culture of endotracheal aspirate secretions. This single available clinical isolate was characterized as Lp1, SBT pattern 3-4-1-1-1-9-1. The pattern corresponded to sequence type 35 (ST35), which we refer to as the outbreak sequence type.
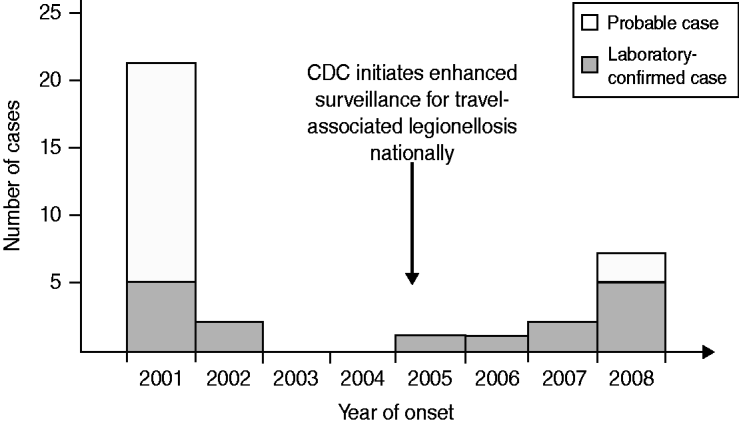
Fig. 2. Number of Legionnaires' disease probable and confirmed cases in travellers to a condominium complex in Las Vegas, Nevada, 2001–2008.
Subsequently, confirmed cases of Lp1 infection were reported in 2002 (two cases), 2005 (one case), 2006 (one case), 2007 (two cases), and 2008 (two cases). Further reporting in 2008, including case-finding via guest notifications, identified three additional confirmed cases and two probable cases in guests who also had stayed overnight at the complex and became ill after departing. The 11 confirmed cases detected after 2001 were all diagnosed by urinary antigen testing.
Epidemiological investigation, 2001
Eighteen (86%) of 21 individuals meeting the case definition for confirmed or probable LD in 2001 were enrolled. Three cases identified after data collection was complete were not included in the case-control study. We enrolled 142 controls after contacting 312 randomly selected, registered guests (response rate 46%). On matched univariate analyses, cases were older than controls (median age 69 years vs. 52 years, P<0·001). Fifteen (83%) of 18 cases and 41 (45%) of 91 controls stayed in tower 2 (mOR 6·1, 95% CI 1·6–22·9). In tower 2, guests who developed LD stayed a median of 7 nights [interquartile range (IQR) 1 night] while unaffected guests stayed a median of 4 nights (IQR 4 nights) (P=0·005). Cases were no more likely than controls to have swum in the pool, used the poolside cabanas or the whirlpool spa, or to have visited the roof of the complex where each was located (Table 1). Furthermore, cases were no more likely than controls to have visited any individual hotel, casino, fountain, or water park in Las Vegas. In the multivariable analyses, cases were significantly (P=0·03) more likely than controls to be in the highest quartile of showering duration (16–90 min/day) compared to the lowest quartile of the same (0–6 min/day) (mOR 23·0, 95% CI 1·4–384) (Fig. 3). Although cases were not significantly more likely than controls to be in the intermediate quartiles of showering duration (>6 to ⩽10 min/day and >10 to ⩽15 min/day), a dose–response trend was noted (mOR 8·5, 95% CI 0·5–153 and mOR 13·2, 95% CI 0·8–217, respectively).
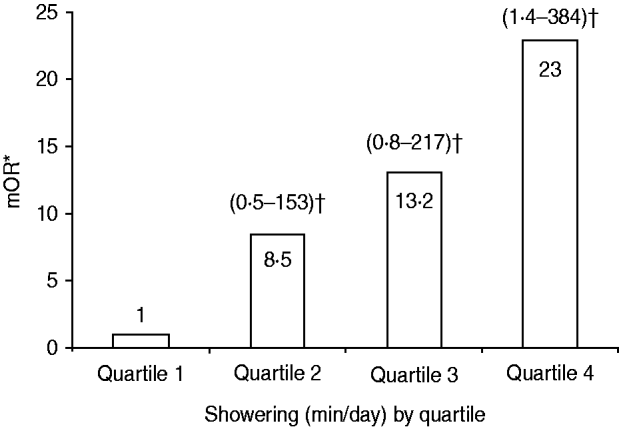
Fig. 3. Multivariate odds ratios for Legionnaires' disease by quartile of showering duration for tower 2 residents, controlling for age, smoking, and underlying medical conditions, Las Vegas, Nevada, 2001. * Matched odds ratios (mORs) for each quartile of showering duration (min/day) vs. quartile 1, calculated using conditional logistic regression. Quartiles are ⩽6 (quartile 1), >6 to ⩽10 (quartile 2), >10 to ⩽15 (quartile 3), and >15 (quartile 4). † Numbers in parentheses represent 95% confidence intervals for mORs.
Table 1. Matched analysis of risk factors and exposures in Legionnaires' disease case and control guests of a condominium complex in Las Vegas, Nevada, 2001
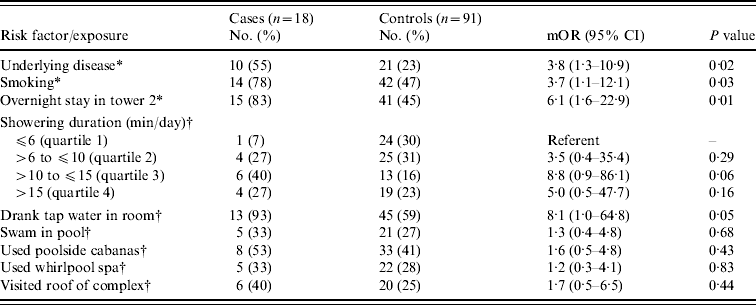
mOR, Matched odds ratio; CI, confidence interval.
* Risk factors assessed for guests of all towers, including presence of ⩾1 underlying conditions associated with increased disease risk [Reference Marston, Lipman and Breiman14] and smoking >100 cigarettes in lifetime.
† Water exposures assessed among tower 2 guests only, including 15 cases and 81 controls except where otherwise specified: drank tap water (14 cases, 76 controls), swam in pool (15 cases, 80 controls), used whirlpool spa (15 cases, 80 controls), and visited the roof of the complex (15 cases, 79 controls).
Environmental investigation, 2001–2002
The time-share condominium is operated continuously throughout the year. The cooling towers located on the roof of the complex had a bromine sanitizer distribution system set for a target level of 1 mg/l. Water features on the roof of tower 2 included a swimming pool, whirlpool spa, and nine cabanas with water misters. A decorative fountain was located in the lobby.
Each tower is served by a low- and a high-rise potable water system; the low-rise water systems are heated by hot-water heaters located on the ground floor, while heaters near the rooftops of the towers serve the high-rise systems. Hence, mid-level floors were distal locations for both the low- and high-rise systems, and sampling of vacant guests' rooms was performed on distal floors that were served by each of the six hot-water systems (Table 2). Specifically, rooms on the 4th and 6th floors of tower 1 were sampled because the first five floors of tower 1 are served by a low-rise system and floors 6–19 are served by a high-rise system. Similarly, rooms on floors 5, 6, and 9 were sampled in tower 2 because floors 1–5 and 6–19 were served by low- and high-rise systems, respectively. In tower 3, floors 8 and 9 were selected for sampling because there are 22 floors in which the first eight floors are served by a low-rise system and floors 9–22 are served by a high-rise system.
Table 2. Sequence-based typing of selected L. pneumophila serogroup 1 isolates from samples collected at a condominium complex, Las Vegas, Nevada, 2001–2008Footnote *
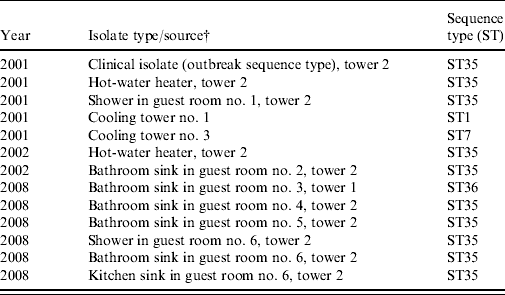
* The complex is composed of a 19-storey central tower (tower 2) and two 18-storey adjacent towers (towers 1 and 3) (Fig. 1). In tower 2, samples were collected from guest rooms near or exactly where guests who developed LD had previously stayed; randomly selected rooms of guests who had not developed LD were selected in towers 1 and 3 (see text).
† L. pneumophila serogroup 1 not detected in samples of rooms in tower 3.
Water temperatures were in the ideal range for Legionella growth (25·0–42·2°C) in both hot and cold water drawn from the taps in rooms where cases stayed. In one guest room, chlorine residuals measured <0·5 and <0·1 mg/l in hot and cold tap water, respectively. Municipal water samples were 17·8–20·0°C in temperature and had 0·7 mg/l of free chlorine residual. Lp1 was isolated from 20 (50%) of 40 samples collected from environmental sources in 2001, including seven (41%) of 17 samples from three guest rooms in tower 2 and 12 (52%) of 23 samples from two cooling towers, the whirlpool spa, and three hot-water heaters that serve tower 2. Other Legionella serogroups and species were also detected in two cooling towers (L. pneumophila, serogroup 13 and L. rubrilucens), the decorative fountain (L. feeleii), and a guest room each in tower 2 (L. feeleii) and tower 3 (L. feeleii). No Legionella was detected in water sampled from the municipal water supply, water heaters in towers 1 and 3, or the cabana misters.
Epidemiological investigation, 2008
The interviews of four patients with confirmed LD and two patients with probable LD for the 2007–2008 case series yielded results that were consistent with the 2001 matched case-control study. The median age of the cases was 70 years (range 54–79 years). Guests who developed LD stayed at the condominium complex a median of 7 nights (range 3–14 nights). All four guests with confirmed LD stayed in an area of tower 2 supplied by the high-rise water heater. Using a list of 35 specific hotels and casinos in Las Vegas, no pattern of frequent visits to any particular location outside the complex was apparent. Five of six cases reported showering in their rooms. Four cases reported smoking histories of ⩾30 years (two cases were current smokers and two other cases were former smokers who had quit in 2007). Two cases also reported diagnoses of bronchitis or emphysema.
Environmental investigation, 2008
Average temperatures of potable water in guest rooms were lower in tower 2 (42·2°C) compared to average temperatures in tower 1 (49·9°C) and tower 3 (48·3°C). Significant differences in chlorine concentrations were not noted in the towers. Seventy environmental samples were collected. Lp1 was isolated from 15 (68·2%) of 22 samples from kitchen and bathroom sink taps or showerheads in four guest rooms of tower 2. In 2008, five selected Lp1 isolates from three of these guest rooms had the same SBT pattern (ST35) as the 2001 clinical isolate (Table 2). Furthermore, retrospective SBT of four environmental isolates collected from guest rooms and water heaters of tower 2 in 2001 as well as 2002 were also found to be ST35. ST1 and ST7 were identified in two cooling towers; ST36 was also found in one guest room in tower 1. Although Legionella was also isolated from one (12·5%) of eight samples from two guest rooms of tower 1 and two (20·0%) of ten samples from two guest rooms of tower 3, the isolate from tower 1 (ST36) did not match the SBT pattern of the outbreak sequence type and the tower 3 isolates were L. feeleii. No Legionella was isolated from 2008 samples of the low- or high-rise water heaters, the water features, or the municipal water.
Remediation and monitoring, 2001–2008
Long-term remediation began with the permanent installation in 2002 of chlorine dioxide injection systems for the disinfection of cold-water supplies in the three towers. Available records documented that chlorine dioxide residual concentrations were initially set to 0·4 mg/l at the point of injection. In 2006, concentrations were increased to <0·8 mg/l (the maximum limit established by Environmental Protection Agency standards) [19]. In 2007, service records indicated chlorine dioxide concentrations within guest rooms of tower 2, which were measured in July (16 rooms) and November (14 rooms), ranged from 0·03–0·6 mg/l. Furthermore, occasional mechanical failures with the chlorine dioxide injection systems (e.g. injection pumps) were documented.
The records review also determined that facility managers and the contracted industrial hygiene firm had frequently detected Legionella colonization in randomly selected guest rooms of tower 2, including during monitoring performed with monthly sampling in 2003 and 2005, quarterly sampling in 2007, and bi-monthly sampling in 2008. They then categorized sampling results into three levels of action based on estimated bacterial concentrations, as ‘system in adequate control’ (<10 c.f.u./ml), ‘prompt action advised’ (10–100 c.f.u./ml), or ‘immediate action advised’ (>100 c.f.u./ml). In response to detection of higher concentrations (i.e. when the system was not in adequate control), prompt or immediate action included closure of guest rooms, implementation of a flushing protocol during a 1-week period, and repeat testing. Sink taps and showerheads were also disinfected following a quarterly schedule. During 2003–2008, positive test results from environmental sampling were communicated to facility managers by the contracted industrial hygiene firm, but these results were not reported to public health. Therefore, public health authorities did not intervene at that time.
In December 2008 (following the second investigation), additional chlorine dioxide generators were installed on the high-rise and low-rise potable hot-water systems of towers 1 and 2. Monthly results of environmental sampling were subsequently reported to SNHD to monitor the effectiveness of remediation of Legionella colonization in the potable water system. In 2009, chlorine dioxide residuals were sustained at close to 0·8 mg/l and hot-water temperatures supplying guest rooms were maintained between 48·8 and 60·0°C. In 2010, improvements to the hot-water system, including thermostatic mixing valves, were engineered to support potable water temperature increases as the primary measure for Legionella remediation. No LD cases in travellers to the condominium complex were detected in 2009 or 2010.
DISCUSSION
From 2001 to 2008, Lp1 colonization of the potable water system caused 16 confirmed and 19 probable LD cases in travellers to a time-share condominium complex in Las Vegas, Nevada. Confirmed cases that were reported after the cluster in 2001–2002 were considered sporadic, and their link to the outbreak in 2001 was not recognized initially. However, following reports of two confirmed cases in 2008, five additional cases were identified by retrospective case-finding. These additional cases demonstrate that, in facilities that have had an outbreak, case-count thresholds for initiating an outbreak investigation (e.g. two or more confirmed cases in a 1-year period) can result in investigation delays and missed opportunities for prevention of LD cases and outbreaks. Despite many other possible exposures to aerosolized water in Las Vegas (e.g. decorative fountains, whirlpool spas), the most significant source of cases' exposures to aerosolized water during their incubation periods was potable water in tower 2 of the complex; the 2001 matched case-control study identified showering as the primary risk factor for transmission. Isolates from samples of the potable water system of tower 2 in 2001, 2002, and 2008 established that the same outbreak sequence type of Lp1 (ST35) colonized this system at the beginning and the end of an 8-year period. Therefore, we hypothesize that ST35 may have persisted in distal areas of tower 2's potable water supply or biofilm (i.e. not necessarily throughout the system), causing ongoing or intermittent levels of LD transmission.
Recurrent LD due to colonized potable water systems has been documented elsewhere [Reference Cowgill20, Reference Kool21]. However, the recurrent transmission in this prolonged outbreak is exceptional because of the unsuccessful long-term remediation and monitoring efforts (e.g. installation in 2002 of chlorine dioxide disinfection systems), which highlight the challenges of complete Legionella eradication in a large facility. In fact, the eradication process often requires an extensive commitment and adjustments may be necessary when setbacks occur. Warm water temperatures [Reference Cowgill20], complex water systems [Reference Kool21], seasonal operations [Reference Mouchtouri22], and older buildings [Reference Borella23] have been previously cited as factors that contribute to Legionella colonization in hospitals and hotels. Many of these same factors probably contributed to colonization at the complex. For example, the condominium's potable water system, which includes six hot-water heaters and low- and high-rise loops for three towers that were built in phases, comprises a complex system. In particular, the high-rise loops in each tower spanned 14 storeys, creating opportunities for reductions in temperature and disinfectant at distal locations serviced by these loops.
The occurrence of this prolonged outbreak is also attributable to the misconception that low Legionella concentrations do not pose a risk for disease. Legionella concentrations were used to establish thresholds for short-term remediation through disinfection of fixtures and flushing in randomly sampled guest rooms where Legionella thresholds were deemed excessive. This focal approach to control can be problematical because contamination of the potable water system is generally a systemic problem; although Legionella amplification may be greatest in distal areas of a system that provides favourable environmental conditions, niche contaminations may be widespread. Although there may be a threshold concentration of Legionella colonization that corresponds with increased risk of infection, there is no known concentration that is safe. Infection may be possible even at low concentrations because numerous factors contribute to transmission, including host susceptibility, bacterial strain characteristics, and type of exposure. Furthermore, a recently published CDC study shows that colony counts of viable Legionella are not reproducible even in the same sample (either among laboratories or within the same laboratory) and can vary by a 3-log range of c.f.u./ml [Reference Lucas24]. Thus, it is in the best interests of public health to consider hazardous the detection of Legionella at any quantitative level, rather than relying on a colony count or cut-off level to take action. Facilities should strive for and maintain undetectable levels of Legionella in the potable water supply and other systems where disease-causing strains have been detected.
SBT is a powerful tool for investigating LD outbreaks. In 2008, the environmental and epidemiological investigations converged because ST35, an uncommon sequence type [Reference Kozak25], was isolated from potable water samples in tower 2 where most guests who developed LD reported showering during their stays; SBT patterns of isolates from the samples collected from tower 2's showerheads and sink taps in 2001, 2002, and 2008 were indistinguishable from the 2001 clinical isolate. Importantly, availability of a clinical isolate is a prerequisite for establishing environmental and epidemiological linkages through SBT. Although guidelines for the diagnosis and treatment of community-acquired pneumonia recommend Legionella testing for patients associated with an outbreak or who have recently travelled [Reference Mandell26], diagnostic tests in this pneumonia cluster were inconsistently used, which led to more probable than confirmed cases. Urinary antigen testing was useful for identifying confirmed cases, but may have precluded culture-based testing. Therefore, patients with pneumonia and a history of travel within 2 weeks of symptom onset should undergo urinary antigen testing as well as culture of respiratory secretions for Legionella [Reference Plouffe27]. Moreover, it has been suggested that ST35 is particularly pathogenic [Reference Harrison and Cianciotto28]. The knowledge that a pathogenic Legionella sequence type has colonized a potable water system or other potential source of transmission should provide further impetus to strive for complete eradication. In the CDC isolate bank, five (0·9%) of 533 clinical isolates of Lp1 that caused cases of sporadic LD are ST35, but ST35 has been associated with four other CDC-supported outbreak investigations involving cooling towers and cruise ships (as of April, 2011).
Although SBT linked the epidemiological and environmental investigations, only a single clinical isolate was available for sequence typing. Epidemiological data should also be interpreted cautiously; water exposures were difficult to distinguish between cases and controls because many of these exposures are common and exposure frequencies and durations were dependent upon respondent recall. Another study was not performed in 2008 (precluding exposure frequency comparisons to a larger population of guests), but data from patient interviews and facility records strongly implicated the route of transmission for the 2007–2008 cluster (i.e. potable water in tower 2). In addition, the case definition used for the 2007–2008 epidemiological investigation refined the case definition used in 2001 by narrowing the incubation period to 2–10 days and replacing hospitalization with antimicrobial treatment effective against Legionella as a probable case criterion, so the case definitions are not perfectly comparable over time. This limitation is offset by an increase in the specificity of the case definition.
This prolonged outbreak illustrates the importance of striving for permanent Legionella eradication in the potable water systems of hotels and other vacation facilities where outbreaks have occurred. The occurrence of a single case after exposure to a facility with a history of an outbreak warrants further investigation by health officials, including a patient interview with a standardized questionnaire and an environmental reassessment at the facility; enhanced communications at all levels of the public health system are necessary to ensure that these activities are accomplished promptly and effectively as essential components of environmental monitoring, case reporting, and surveillance for travel-associated LD. Recurrent LD in facility guests also signals the need for enhanced or alternative remediation measures and presents a disease prevention opportunity.
ACKNOWLEDGEMENTS
We gratefully acknowledge the guests and management of the condominium complex for their participation in this investigation. We also thank the numerous epidemiologists, environmental health specialists, and microbiologists at CDC and the Southern Nevada Health District for their contributions to these investigations. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support was received from the U.S. Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.







