INTRODUCTION
Salmonellosis is a common cause of foodborne diarrhoeal disease worldwide. The majority of infections are transmitted from healthy carrier animals to humans via contaminated food. The main reservoir of zoonotic Salmonella is food-producing animals and the main sources of infections in industrialized countries are animal-derived products, notably fresh meat and poultry products. Table eggs were until 2004, the most important food source of human salmonellosis in Denmark [1, Reference Hald, Smulders and Collins2].
The Salmonella control programme in the table-egg production was implemented in Denmark in December 1996 and has been revised regularly. The objective is the complete eradication of Salmonella enterica in the commercial table-egg sector including barnyard sales.
The programme is based on the principle of top-down eradication. All flocks destined for table-egg production are monitored by a combination of serological and bacteriological testing to ensure early detection [Reference Feld3, Reference Skov4]. Testing frequency and materials are shown in Table 1. Following positive routine samples, the veterinary authorities collect additional official samples in the flock for bacteriological and serological verification. Verified infected flocks are restricted by public order regarding movement of flocks outside the farm premises and specific hygienic procedures are required. Breeder and rearing flocks infected with S. Enteritidis and S. Typhimurium are culled according to the EU Zoonosis Directive (92/117/EEC), but flocks infected with other serotypes are also usually destroyed.
Table 1. Salmonella control programme for table-egg production in Denmark 2006

* Requirements of the European Union Zoonosis Directive (92/117/EEC).
† Samples taken by the District Veterinary Officer.
‡ Samples taken by the District Veterinary Officer every 8 weeks.
§ Samples taken by the District Veterinary Officer every 3 months.
Initially, all commercial layer flocks infected with S. Enteritidis and S. Typhimurium were culled. But as the occurrence of Salmonella was so extensive, that the supply of eggs to the Danish market would be significantly reduced if all infected layer flocks were culled, this strategy was terminated in September 1997. From March 1998, layer flocks testing positive in the monitoring programme are only allowed to sell their production for heat-treated egg products. Even though only one of several flocks on a farm tests positive, pasteurization of eggs from all flocks can be required.
The reduction of Salmonella in table-egg layers is achieved primarily by eradication of infected breeder flocks, but also by increased hygiene and bio-security measures at hatcheries and in layer farms [Reference Hald, Smulders and Collins2].
The proportion of layer flocks infected with Salmonella, notably S. Enteritidis, has been markedly reduced since the initiation of the programme [Reference Wegener5]. Since the initial culling of infected central rearing and breeder flocks in 1997 (7/38 tested flocks), no infections have been detected at this level in the production pyramid, whereas 1–4% of the pullet-rearing flocks were found to be infected during 1998–2002. The proportion of infected table-egg-producing layer flocks has decreased from 13·4% in 1998 to 2·6% in 2002 and 0·4% in 2006 [1, 6]. Major reductions on the incidence of foodborne human salmonellosis have also been reported [1].
It is not without costs to ensure safer food products. The Salmonella control programme for table eggs has generated substantial costs for the Danish table-egg producers and the public sector. These costs relate to monitoring and control as well as research and administration. The main societal benefit from increased food safety is improved health of the population, resulting in reduced health-care costs (hospitals, local physicians, tests, etc.) and increased productivity arising from fewer days of illness. However, the industry also benefits from consumers increased trust in their products.
In this paper, we describe the effects, costs and benefits of control of Salmonella in Danish table-egg production.
MATERIALS AND METHODS
To estimate the effects, costs and benefits of the control programme, the following questions must be answered:
(1) How many human Salmonella cases are egg-associated?
(2) How many egg-associated cases have been avoided due to control?
(3) What are the societal costs of human salmonellosis?
(4) What are the (public) costs of control?
Estimating the number of egg-associated cases
In Denmark, all laboratory-confirmed human cases are reported to a central database. In order to estimate the number of human Salmonella infections attributable to the various food animal sources, a stochastic model was developed. The principle is to compare the reported number of human cases caused by different subtypes with the distribution of the same subtypes isolated from the different animal reservoirs or food sources. It is a prerequisite that some of the dominant subtypes are found almost exclusively in a single source. Such subtypes are regarded as indicators for the human health impact of that particular source, assuming that all human infections with these subtypes originate only from that source. Human infections caused by subtypes found in several sources are then distributed relative to the prevalence of the indicators. This approach requires integrated surveillance of the pathogen in most major food animals, food (including imported food) and humans, providing a collection of representative isolates from the farm-to-fork chain, followed by the use of appropriate discriminatory typing methods. The model has been described in detail by Hald et al. [Reference Hald7]. Total number of reported cases and number of reported cases attributable to Danish table eggs during 1997–2006 are presented in Table 2.
Table 2. Mean number of human Salmonella cases, assessed as being attributable to the consumption of Danish table eggs, and the total number of reported cases from 1997 to 2006
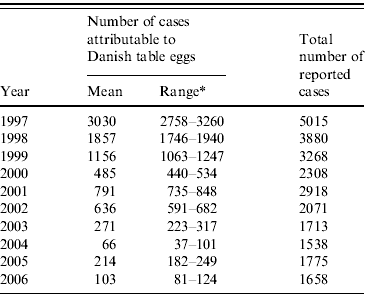
Mean=MeanN i and range=MinN i and MaxN i in Table 3.
* Estimated 95% credibility limit.
Estimating (guesstimating) the number of avoided cases due to control
Assuming that the number of human Salmonella cases had remained at the same level as in 1997, as if no control programme had been implemented, the number of avoided cases was estimated by subtracting the annual number of egg-associated cases (estimated as described above) from the number of egg-associated cases in 1997. The expected number of reported cases without control was modelled using the estimated number of egg-related cases in 1997, assuming a range of ±20%. The number of avoided cases was estimated for the reported and unreported cases, assuming that 10% (range of 5–20%) of all human Salmonella infections were reported. Data and distributions used in the probabilistic modelling are presented in Table 3.
Table 3. Description of model parameters

GP, General physician.
Note that the costs assumed avoided due to implementation of the control plans, are estimated by replacing N ik with A ik in the equations.
† Sector of employment: P, private; C, central government; L, local government.
‡ Data on all hospitalized patients diagnosed with Salmonella 1991–1998 [Reference Helms, Simonsen and Mølbak9].
§ Proportion of false-negative diagnoses is based on data from 46 reported outbreaks in Denmark during 1997–2002, where more than one person was reported sick (bootstrapped mean and standard deviation).
∥ Proportion of cases consulting a GP based on data from Rosdahl & Schmidt [Reference Rosdahl and Schmidt10].
Estimating the societal costs of human salmonellosis
The societal cost due to zoonotic Salmonella infection in 2001 has been estimated by Korsgaard et al. [Reference Korsgaard, Wegener and Helms8]. The costs were estimated for seven different patient groups: hospitalized cases that underwent surgery (group 1), with invasive infections (group 2) or without complications (group 3), cases diagnosed by a general physician (GP) (group 4), cases with a false-negative GP diagnosis (group 5), cases with no GP diagnosis (group 6) and cases that stayed at home (group 7). Patient groups 5–7 constitute the unreported cases. Assumed health-care cost per case in the seven patient groups in 2001 are presented in Table 4.
Table 4. Assumed health-care costs per case in 2001 and estimated proportion of total cases for each patient group
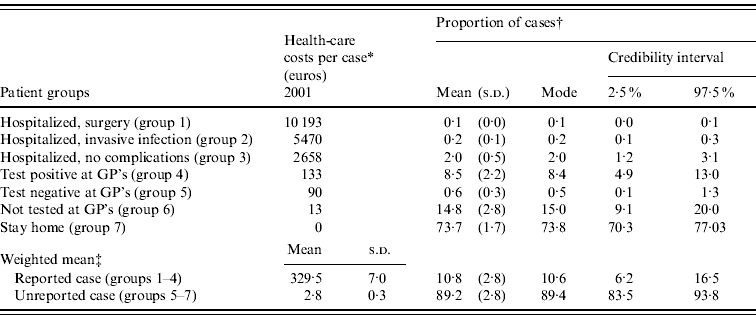
GP, General physician.
* Baseline estimates based on 2001 costs from Korsgaard et al. [Reference Korsgaard, Wegener and Helms8]. Assuming a 2% rate of inflation per year. Cost per case=h j in Table 3.
† Based on Monte Carlo simulation, 10000 iterations (see Table 3 for description of model parameters).
‡ Weighted mean is the sum of ‘the proportion of cases multiplied by the health-care cost per case’ based on the 10 000 iterations (mean=![]() hk and s.d.=
hk and s.d.=![]() hk in Table 3).
hk in Table 3).
Treatments for all hospitalized patients are registered in a national database, and during 1991–1998, 25·9% of the cases diagnosed with Salmonella were hospitalized [Reference Helms, Simonsen and Mølbak9]. Of these, 2·4% underwent surgery (group 1) and 8·1% had an invasive infection (group 2). The rest of the reported cases were assumed diagnosed by a GP (group 4).
Sensitivity of laboratory tests is not 100%, so some of the truly infected cases will not be diagnosed when tested at a GP's. Based on data from outbreak reports, it was estimated that the risk of receiving a false-negative diagnosis was 6·2% (bootstrap of data from 46 outbreaks).
Only a minor proportion of the cases consulted a GP. A telephone survey conducted in 1992 [Reference Rosdahl and Schmidt10] showed that only 24% of Danish households, where one or more persons had experienced diarrhoea during a 3-month period, contacted a GP. The proportion of cases consulting a GP without being tested (group 6) was estimated as 24% minus the proportion of cases that were tested at a GP's (groups 4 and 5).
Distributions used in the probabilistic modelling are presented in Table 3.
The costs of lost labour were estimated by assuming 14–21 days of illness for each hospitalized patient and 10–14 days of illness for cases tested at a GP's [Reference Mølbak11]. Data on number of days of illness were not available for cases not tested at a GP's or cases that stayed at home, but we assumed as 1–4 days of illness. The number of days of illness was modelled by uniform distributions, and 61% were assumed to be work days (Table 5).
Table 5. Assumed days of illness and mean number of lost work days per case
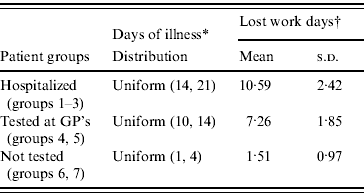
GP, General physician.
* Based on expert opinions and Mølbak et al. [Reference Mølbak11].
† Assume 221 work days per year, modelled as Binomial (days of illness 221/365). Monte Carlo simulation, 10 000 iterations (mean=![]() dj and s.d.=
dj and s.d.=![]() dj in Table 3).
dj in Table 3).
The proportions of cases with lost production per year were assumed to be the proportion of adults (aged>18 years) in employment, thus assuming that adults stayed home to take care of sick children. The employed cases were split into three groups, proportional to the number of persons employed in the private sector or in central and local government each year. The salary per day was modelled by Pert distributions using reported salary lower-quartile, median and upper-quartiles for each sector, assuming a 7·5-h work day (Table 6). Data regarding employment and salary was obtained from Statbank Denmark [12].
Table 6. Proportion of cases with lost production and costs per lost work dayFootnote * for persons working in the private and government sectors in DenmarkFootnote †

We applied the cost model for the ‘avoided’ egg-associated cases assuming that these infections would have been distributed among the different patient groups proportionally to the actual number of infections. The average health-care cost and lost production per case was estimated, assuming a 2% rate of inflation per year.
Monte Carlo simulation models were set up in @Risk 4.5 from Palisade Corporation (Newfield, NY, USA) (Latin Hypercube sampling, seed=1, iterations=10 000).
The public costs of Salmonella control
From the Danish Veterinary and Food Administration, we received a statement of the costs for control in the period from 1997 to 2002. The costs were divided into costs used for surveillance and costs used to compensate farmers that had to buy replacement stock due to Salmonella infection (Table 4). For S. Enteritidis and S. Typhimurium, the EU reimburses 50% of the costs of replacement of breeding flocks. Unfortunately since 1999, the costs regarding surveillance in both the broiler and table-egg sector were accounted together meaning that we did not have the exact surveillance costs related only to the table-egg production. Consequently, we estimated this proportion based on the costs for 1998. Since 2001, the routine sampling costs are assumed by the industry, which leads to a significant decrease in the overall public costs in 2001 and 2002. By right, these costs should be included in the cost estimates, but we did not have access to these figures.
RESULTS
Table 2 shows the estimated number of cases of salmonellosis attributable to the consumption of Danish table eggs between 1997 and 2006. The decreasing tendency in the total number of cases, as well as on the percentage of cases attributable to table eggs, is significant. In 1997, 5015 cases of human salmonellosis were reported. Of these, 55–65% was estimated to be associated with eggs. In 2002, 29–33% of the total 2071 cases were related to the consumption of eggs. Nevertheless, in 2002 Danish table eggs were still the most important source of human Salmonella infections in Denmark. In 2006, only 5–7% of the total 1658 cases were assumed to be related to the consumption of eggs.
The model estimated that during the period 1998–2002, the control programme had avoided 10 200 (95% CI 8100–12 400) reported cases. Figure 1 shows the estimated number of avoided cases per year assuming that probably 10% are reported and that the number of egg-related cases without the Salmonella control programme had remained at the 1997 level of about 3030 reported cases. Including the uncertainty related to the degree of underestimation (range of 5–20%) the total number of avoided cases during 1998–2002 was estimated to be 106 600 (95% CI 67 000–181 500).
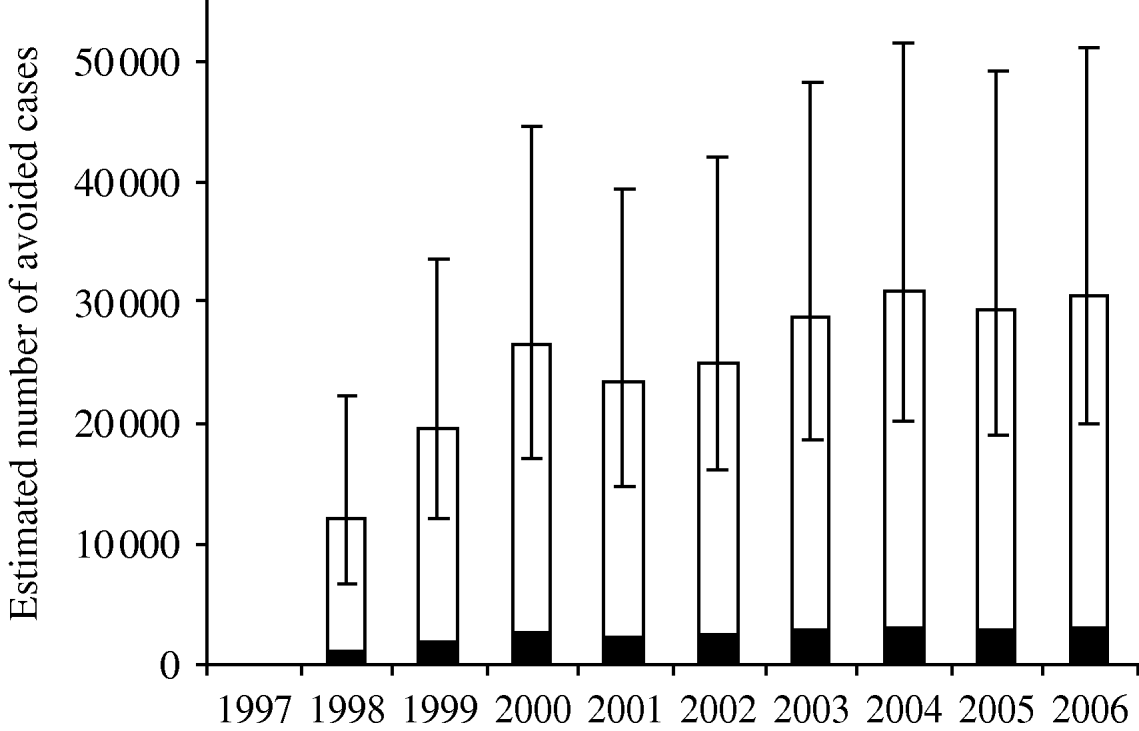
Fig. 1. Estimated most-likely number of avoided reported (■) and unreported (□) Salmonella cases per year. Assuming a probable reporting fraction of 10% (range 5–20%) and that the number of egg-related cases had remained at the 1997 level as in case of no control. Error bars indicate 95% credibility intervals for the total number of avoided cases per year.
The cost model estimated that during the period 1998–2002, the control programme has probably reduced the societal costs to health care and lost production by 9·5 million euros (95% CI 7·6–11·4) for the reported cases. Figure 2 shows the estimated avoided societal costs per year assuming that probably 10% of the cases were reported. Including the uncertainty related to the degree of underestimation the probable avoided societal costs during 1998–2002 were estimated to be 21·1 million euros (95% CI 15·2–30·6).
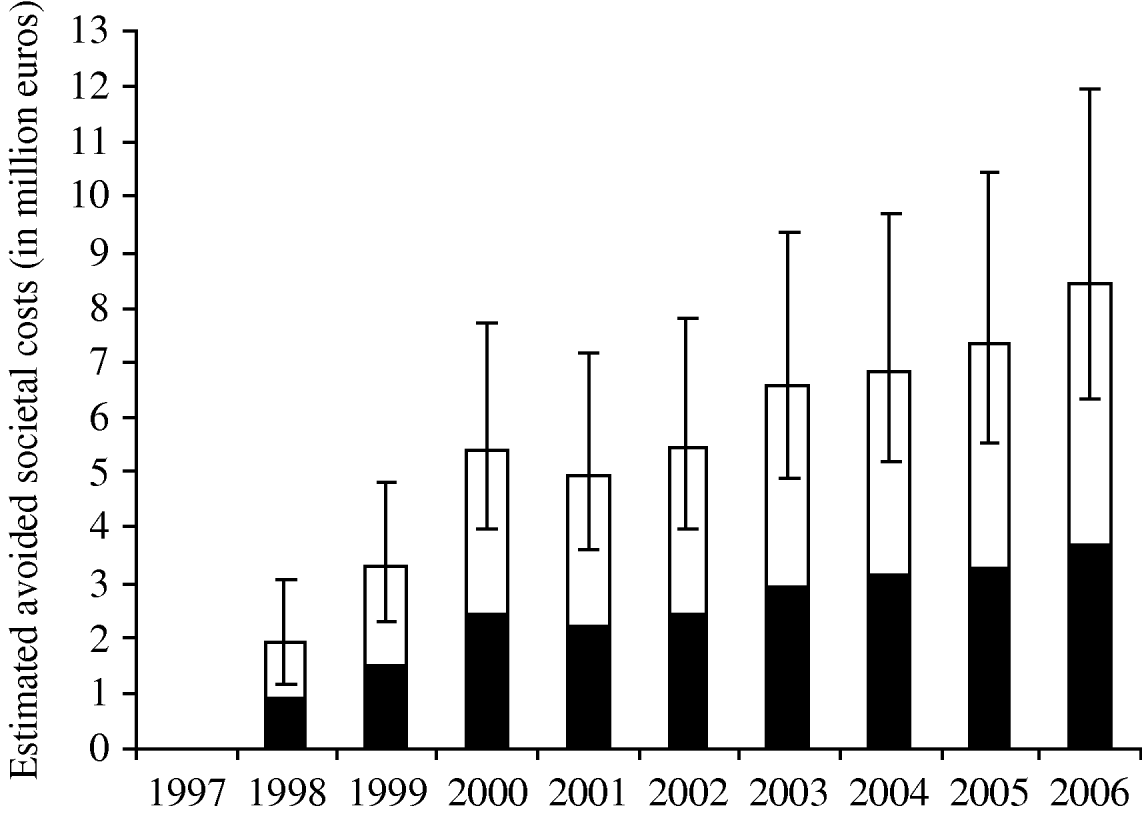
Fig. 2. Estimated probable avoided societal costs (in million euros) per year for reported (■) and unreported (□) Salmonella cases. Assuming a probable reporting fraction of 10% (range 5–20%) and that the number of egg-related cases had remained at the 1997 level as in case of no control. Error bars indicate 95% credibility intervals for the total number of avoided cases per year.
Finally, based on the public cost of control (Table 7) and the estimated avoided societal costs due to control, the cost–benefit ratio per year was calculated (Fig. 3). The results showed a continuous decreasing cost–benefit ratio reaching well below 1 in 2002. For the period 1997–2002, the mean cost–benefit ratio was 0·5.

Fig. 3. Annual ratios of the public costs of control and the estimated avoided societal costs. Estimated costs in 2001 and 2002 do not include the industry's costs of routine sampling.
Table 7. Public costs of the Danish Salmonella control programme in table-egg production from 1997 to 2002
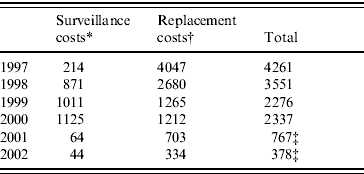
Values are given ×1000 euros.
* From 1999, the surveillance costs for the broiler and table-egg sector were accounted together. The costs for the table-egg production were estimated based on the proportion observed for 1998.
† EU reimburses 50% of the costs due to replacement stock in breeding flocks.
‡ Does not include the industry's costs of routine sampling.
DISCUSSION
We assumed that if no control programme had been implemented, the number of human salmonellosis cases attributable to Danish eggs would have remained at the 1997 level. This is probably a very unlikely scenario. Some would probably argue that the prevalence of Salmonella among egg-layers, and consequently the human incidence, would have continued to increase. On the other hand, the human incidence in several EU countries and the United States appeared to decline towards the end of the 1990s [13, Reference Patrick14]. This suggests an overall decreasing trend, which may be due to an increasing awareness of zoonotic bacteria in production animals. A positive effect of implementing national control programmes and the EU directives regarding control of Salmonella in breeding flocks of Gallus gallus were observed in several EU countries [13]. However, no countries experienced such a rapid decrease as the one observed in Denmark [1].
In this model, due to lack of Danish data, we assume that about 10% of the Salmonella-infected cases are reported. However, it has been estimated that about 3% of the zoonotic Salmonella cases were reported in the United States [Reference Voetsch15] compared to an estimated 32% in England [Reference Weeler16]. If including the English estimates of the proportion of Salmonella-infected cases consulting a GP (71%) and the overall proportion of cases reported (32%), the probable total number of avoided cases during the period 1998–2002 would be reduced from 106 600 to 41 300 and the probable avoided societal costs would be reduced from 21·1 to 13·6 million euros. As a result, the mean cost–benefit ratio for the period 1997–2002 would increase from 0·5 to 0·7.
The estimate of the avoided societal costs must be considered a minimum. Primary costs to medicine and non-medical cost such as transportation have not been included. Additionally, and more importantly, the cost of other complications requiring special care, sequelae and premature death were not included.
By right, costs of control in 2001 and 2002 should have included the costs of routine sampling. However, these costs were assumed by the industry at this time and we did not have access to them. However, judging from Figure 3, the cost–benefit ratios probably did not exceed 0·5 in 2001 and 2002 even if the costs of routine sampling had been included.
By the end of 2002, public financing ended and the poultry industry took over the administrative and financial responsibility of the programme. Therefore, information regarding industry costs in 2003 was not available, but the estimated avoided costs (Fig. 2) suggest that the cost–benefit ratio will continue to decrease over the next years, which is a logical effect of the continuing reduction of infected table-egg layer flocks.
CONCLUSION
The Danish Salmonella control efforts in table-egg production have been successful in achieving their main objective: reducing the number of egg-associated human cases of salmonellosis. From a societal point of view, the results presented here also indicate that the control efforts have been a good investment. Table eggs are, however, still one of the most important domestic food sources of human salmonellosis in Denmark. The aim must therefore be a continuing reduction in the primary production, based on effective surveillance and control.
DECLARATION OF INTEREST
None.












