Introduction
Influenza epidemics follow seasonal patterns that vary in distribution and severity and are associated with excess morbidity and mortality. Worldwide, these annual epidemics are estimated to result in about 3 000 000–5 000 000 cases of severe illness, and between 300 000 and 500 000 deaths [1, 2]. Pregnant women, small children, elderly people and anyone with medical risk conditions are at higher risk for severe infection and death. In industrialised countries, hospitalisation and death occur mainly in high-risk groups and most influenza-related deaths occur in people aged ⩾65 years. Therefore, influenza prevention and control remains a major challenge for public health systems worldwide [Reference Treanor, Bennet, Dolin and Blaser3].
Antiviral treatment with neuraminidase inhibitors (NAI) is considered an important adjunct to vaccination in order to reduce the risk of severe illness due to influenza among adults, particularly those with underlying medical risk conditions. Since the 2009 influenza A (H1N1) virus pandemic, both the United States Advisory Committee on Immunization Practices and the WHO recommend early, empirical antiviral treatment for those with suspected or confirmed influenza requiring hospitalisation or who have severe, progressive or complicated illness [2, Reference Harper4]. However, there are limited data on adherence to these recommendations in clinical practice [Reference Spagnuolo5], particularly among high-risk populations [Reference Lindegren6].
In Catalonia (Spain), antiviral treatment should be administered only to hospitalised-confirmed cases of severe influenza and to hospitalised patients at risk of severe complications. Groups at high risk of complications include pregnant women and people with underlying medical conditions such as chronic lung disease (including asthma, cystic fibrosis and lung dysplasia); chronic cardiovascular disease (excluding hypertension); type I and type II diabetes; moderate–severe kidney impairment; haemoglobin and other haematologic disorders; liver impairment; immunosuppressive disorders; severe neuromuscular disorders and patients with morbid obesity (body mass index (BMI) >40) [7]. Antiviral treatment is not administered in primary care centres.
The efficacy of NAI in adults with risk factors for influenza complications has not been extensively evaluated [Reference Treanor, Bennet, Dolin and Blaser3]. Although a randomised clinical trial (RCT) confirmed that NAI reduce symptoms, no RCTs have examined the effectiveness of NAI against more serious outcomes [Reference McGeer8]. However, an RCT does not fully inform on the effectiveness of a product as used in real clinical practice [Reference Martin9].
A systematic review of NAI by Michiels et al. concluded that there is no evidence of treatment benefits in elderly and at-risk individuals, vaccinated or not, on relevant outcomes such as hospitalisation and mortality [Reference Michiels10].
In a meta-analysis by Hsu et al. of observational studies in any population that compared antiviral drugs with no antiviral treatment, earlier treatment was associated with significantly better outcomes in terms of avoiding hospitalisation and intensive care unit (ICU) admissions [Reference Hsu11].
The suboptimal use of NAI in high-risk patients and the lack of confidence in their effectiveness in healthcare workers justify the need for studies on the effectiveness of antivirals on meaningful clinical endpoints in high-risk patients [Reference Lindegren and Schaffner12].
In October 2010, the Public Health Agency of Catalonia initiated the surveillance of severe hospitalised cases of influenza as a tool to complement information provided by the influenza sentinel system based on primary healthcare physicians. The objectives of this study were to investigate predictors of antiviral treatment in severe hospitalised influenza cases during six influenza seasons and the effect of early antiviral treatment in avoiding death.
Material and methods
Study design
We carried out an observational epidemiological study of the effect of NAI treatment in adult patients hospitalised due to severe acute respiratory influenza virus infection.
In 2010, a surveillance system for severe influenza was started in Catalonia, a region in the northeast of Spain with 7.5 million inhabitants, in order to (a) estimate the severity of seasonal influenza epidemics and their impact on health services according to the virological characteristics of influenza; (b) provide information to improve influenza prevention and control; and (c) identify risk groups for severity. The system includes 12 hospitals covering a total population of 4 644 543 (62% of the Catalan population) that report on hospitalised cases of confirmed severe influenza in each influenza season. Epidemiological surveillance of severe hospitalised cases of influenza in Catalonia begins in the 40th week of the year and lasts until week 20 of the following year: the participating hospitals report severe hospitalised influenza cases to the corresponding epidemiological surveillance unit [13].
A severe hospitalised influenza case was defined as a severe case of laboratory-confirmed influenza virus infection that required hospitalisation (pneumonia, septic shock, multiorgan failure or any other severe condition, including ICU admission) or who developed clinical signs during hospitalisation for other reasons. The diagnosis was confirmed by PCR and/or culture of nasopharyngeal swabs [14].
Respiratory tract samples were processed at each hospital laboratory within 24 h of receipt. A 300 µl aliquot was taken for total nucleic acid extraction and eluted in 25 µl of RNase-free elution buffer using the automatic QIAsymphony system (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Subsequently, two specific one-step multiplex real-time PCR using Stratagene Mx3000P QPCR Systems (Agilent Technologies, Santa Clara, CA, USA) were carried out for typing A/B influenza virus (sensitivity was 10 and 103 copies/μl, respectively) and subtyping influenza A virus (sensitivity was 102, 103 and 10 copies/μl for H1, H3 and H5 RNA, respectively) [Reference Suwannakarn15].
Data collected
Reported cases of laboratory-confirmed severe hospitalised influenza in persons aged ⩾18 years during six influenza seasons (2010–2011 to 2015–2016) were included.
For each reported case, we recorded the variables age, sex, chronic obstructive pulmonary disease (COPD), asthma, obesity (BMI >40), chronic renal disease, immunodeficiency (HIV infection or other), chronic cardiovascular disease, chronic liver disease, pregnancy, ICU admission, date of symptom onset, complications (secondary or primary pneumonia, acute respiratory distress syndrome and multiple organ failure), death, type of virus (A, B or C), seasonal influenza vaccination status and date and drug of NAI treatment.
Cases were considered vaccinated with the influenza vaccine if they had received a dose of the vaccine ⩾14 days before symptom onset.
The information for each study variable was collected by public health officers of the surveillance units of Catalonia through an epidemiological survey. The primary source of information was the medical record.
Statistical analysis
The demographic, virological and clinical characteristics of treated and untreated patients were compared using the χ 2 test.
Associations between death and the independent variables, including NAI treatment (early treatment and late treatment compared with no treatment), were assessed in a bivariate analysis. Possible interactions between antiviral treatment and independent variables were analysed by logistic regression. Independent variables were checked for collinearity using the variance inflation factor [Reference Katz16].
Because the participant hospitals may not be homogeneous and there were differences in the number of deaths between hospitals, a mixed-effects logistic regression model with the variable hospital as a random intercept was constructed to estimate the crude and adjusted odds ratio (aOR) and their corresponding 95% confidence interval (CI). To calculate the aOR, a multivariable analysis was made using the propensity scores, which were estimated by logistic regression with NAI treatment as the outcome and age, sex, COPD, asthma, obesity, chronic renal disease, immunodeficiency, chronic cardiovascular disease, chronic liver disease, pregnancy, seasonal influenza vaccination, type of virus and mismatches between circulating influenza strains and the components of the seasonal vaccine as independent variables. The propensity score was used as a continuous covariate in a final mixed-effects logistic regression model.
The analysis was performed using the SPSS v.24 statistical package and the R v3.3.0 statistical software (http://cran.r-project.org).
Ethical considerations
All data used in the analysis were collected as part of the routine public health surveillance activities and were therefore exempt from the institutional review board review.
Results
A total of 1727 hospitalised patients aged ⩾18 years were included during the study period, of whom 91.3% received NAI (oseltamivir 99.7%, zanamivir 0.26%). Demographic and clinical characteristics and influenza vaccination status are shown in Table 1. Patients aged 18–64 years, patients with respiratory distress syndrome, patients admitted to the ICU and patients who survived had the highest frequencies of NAI treatment.
Table 1. Characteristics of influenza cases treated and untreated with neuraminidase inhibitors, Catalonia, 2010–2016
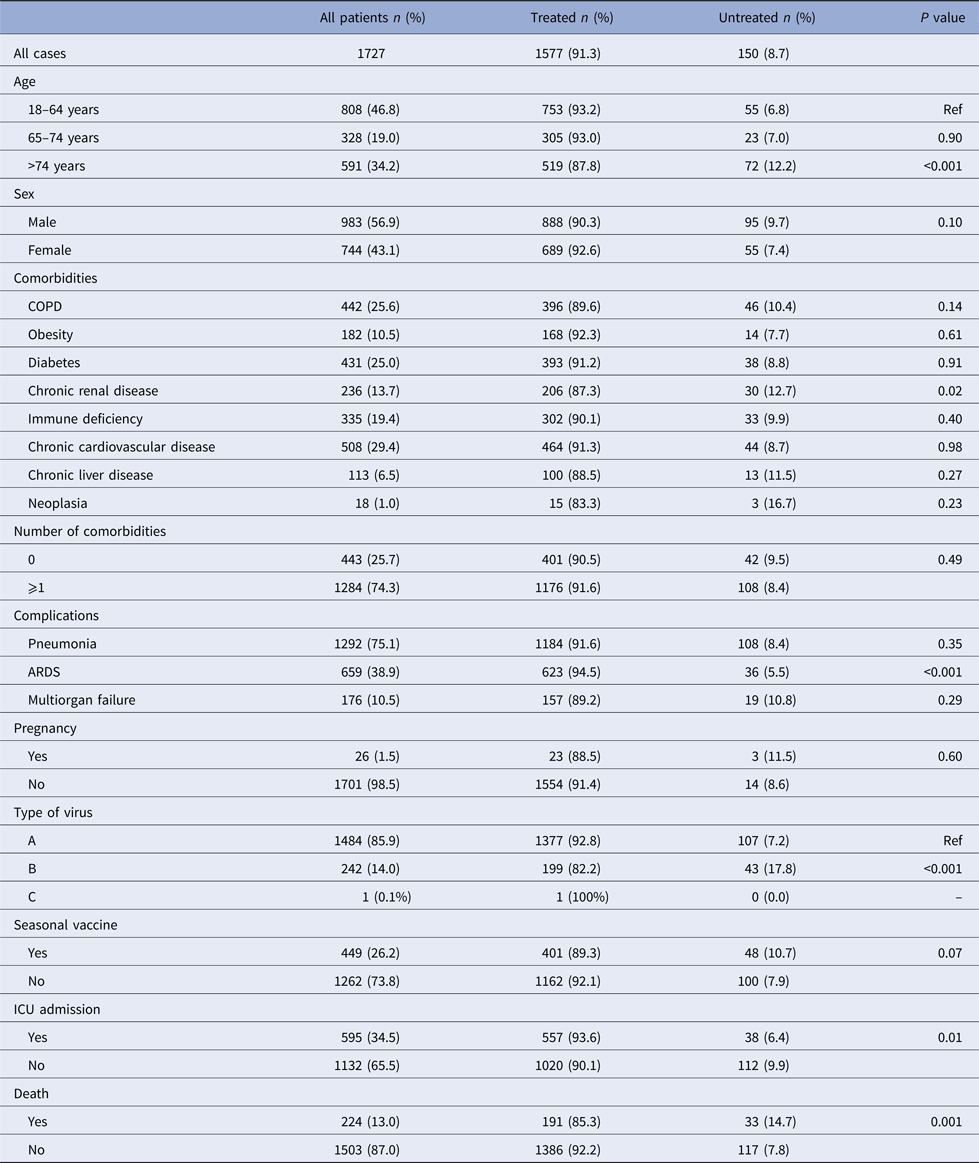
ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
The frequency of treatment by season is shown in Figure 1: the highest frequency of NAI treatment (95.3%) was in the 2010–2011 season and the lowest (78%) in the 2012–13 season.
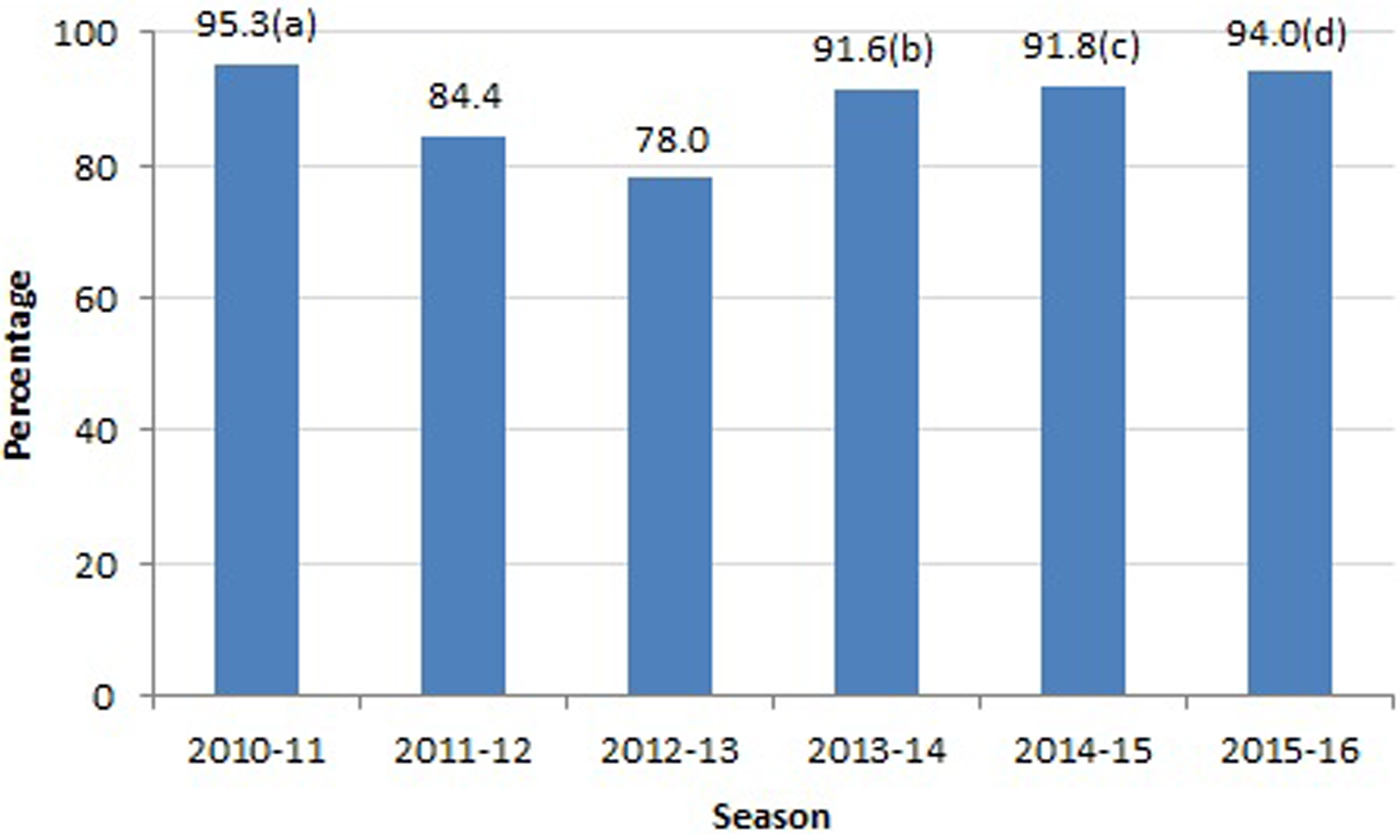
Fig. 1. Frequency of NAI treatment according to influenza season. NAIs, neuraminidase inhibitors. (a) Highest values were observed for influenza A, NO multiorgan failure and survival patients. (b) Highest values were observed for NO chronic renal failure. (c) Highest values were observed for influenza A, acute respiratory distress syndrome, NO pneumonia and survival. (d) Highest values were observed for influenza A, acute respiratory distress syndrome and survival.
A total of 437 patients (26.3%) received NAI treatment within 48 h of symptom onset, 649 (39.1%) within 3 days and 1001 (60.3%) within 5 days.
The seasons with mismatch were 2014–15 for the influenza A virus and 2011–12, 2013–14 and 2015–16 for the influenza B virus.
No interaction was found between NAI treatment and the other variables investigated, and there was no collinearity between the variables.
Death occurred in 224 patients (13%): in the bivariate analysis, death was associated with age ⩾65 years, chronic renal disease, immunodeficiency, chronic cardiovascular disease, chronic liver disease and not receiving NAI (Table 2). In the multivariable analysis, factors associated with a reduction in deaths were: NAI treatment (aOR 0.56, 95% CI 0.36–0.86), NAI treatment ⩽48 h after symptom onset (aOR 0.37, 95% CI 0.22–0.63), NAI treatment ⩽3 days after symptom onset (aOR 0.49, 95% CI 0.30–0.79) and NAI treatment ⩽5 days after symptom onset (aOR 0.50, 95% CI 0.32–0.79) with respect to those who did not receive treatment (Table 4).
Table 2. Factors associated with death in hospitalised patients, Catalonia, 2010–2016
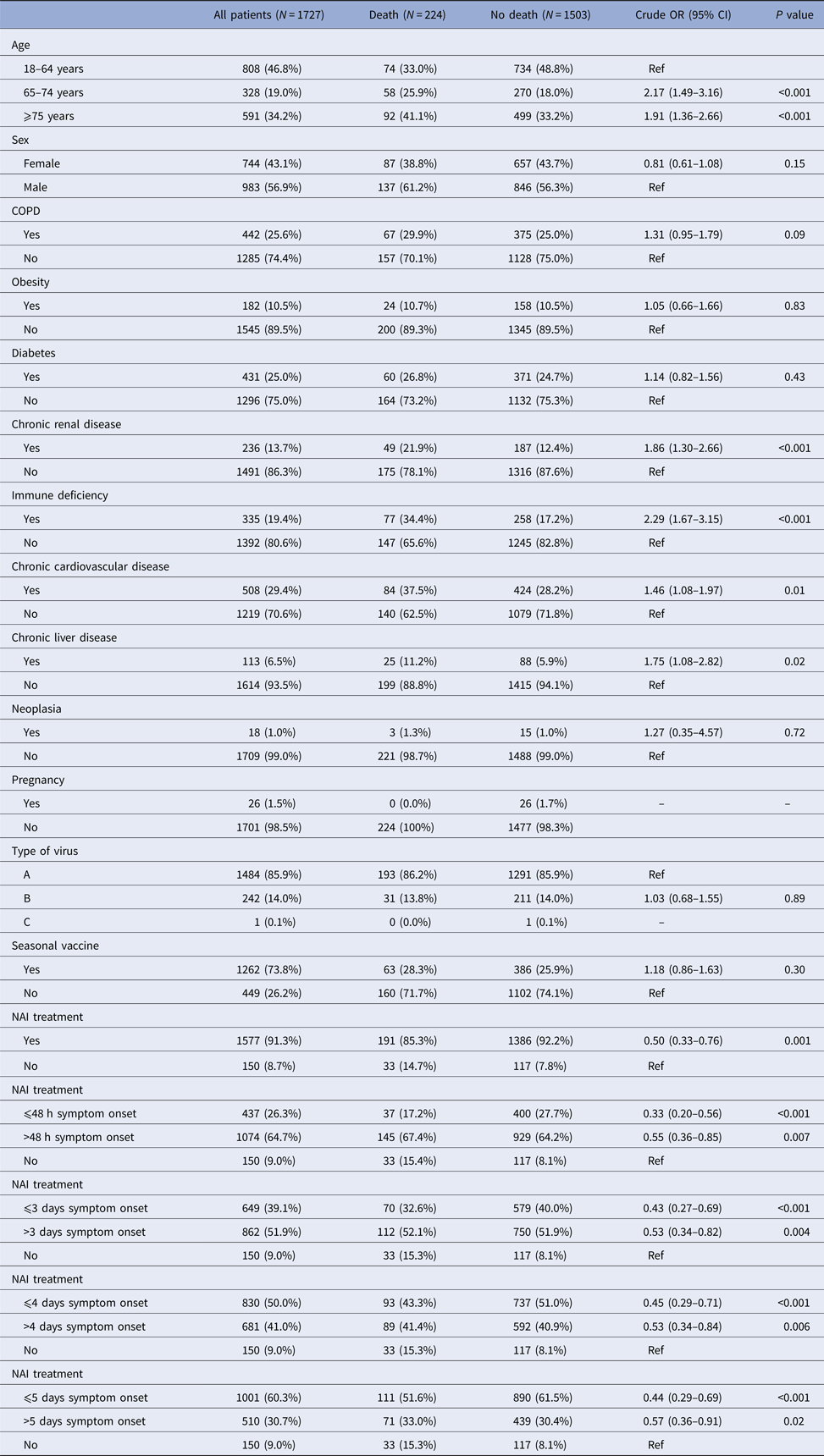
NAI, neuraminadase inhibitors.
Of the 595 patients who required ICU admission, 128 (21.5%) died: in the bivariate analysis, death was associated with age ⩾65 years, chronic renal disease, immunodeficiency, chronic cardiovascular disease, chronic liver disease and not receiving NAI treatment (Table 3). In the multivariable analyses, NAI treatment ⩽48 h after symptom onset (aOR 0.32, 95% CI 0.14–0.74), NAI treatment ⩽3 days after symptom onset (aOR 0.44, 95% CI 0.20–0.97) and NAI treatment ⩽5 days after symptom onset (aOR 0.45, 95% CI 0.22–0.96) with respect to those who did not receive treatment were associated with a reduction in deaths. Treatment >5 days after symptom onset was not associated with a reduction in deaths in all hospitalised patients (aOR 0.63, 95% CI 0.39–1.02) or in patients admitted to the ICU (aOR 0.60, 95% CI 0.28–1.29) (Table 4).
Table 3. Factors associated with death in patients admitted to the intensive care unit, Catalonia, 2010–2016

NAI, neuraminidase inhibitors.
Table 4. Crude and adjusted OR of NAI treatment in reducing the mortality in hospitalised patients and patients admitted to the intensive care unit, Catalonia, 2010–2016

NAI, neuraminidase inhibitors.
The distribution of antiviral treatment, deaths and ICU admission by hospital and by season is shown in Supplementary Tables S1 and S2 and the crude OR and aOR of NAI in reducing deaths in different subgroups of patients are shown in Supplementary Table S3.
Discussion
This study, based on the surveillance of severe hospitalised patients with laboratory-confirmed influenza in the 2010–2011 to 2015–2016 seasons in Catalonia, found a high frequency of patients receiving NAI and suggests that NAI treatment was effective in reducing the risk of death. The results also suggest a certain dose–response relationship between the effectiveness of NAI treatment in avoiding death and the time from symptom onset to the initiation of NAI treatment.
The frequency of hospitalised patients who received NAI treatment during the whole period was 91.3%, slightly higher than the 86% observed in a study of adults hospitalised with laboratory-confirmed influenza in the USA in the 2010–2011 to 2014–2015 seasons [Reference Appiah17] and the 70% observed in another Spanish study in the 2010–2011 season [Reference Fernández18], but lower than the 96.2% of hospitalised patients in the Japanese study by Maruyama et al. during the 2010–2013 influenza seasons [Reference Maruyama19]. In Japan, NAI is recommended for patients with a positive rapid diagnostic antigen test, which may explain the high level of treatment adherence.
We found that 93.6% of patients admitted to the ICU received NAI, very close to the 94.8% found in a US study during the 2013–2014 season [Reference Shah20] and higher than the 85.8% observed in patients of all ages admitted to the ICU in China by Xu et al. in the 2010–11 season. We observed a decrease in the proportion of patients who began NAI treatment within 2 days after onset symptom according to disease severity (26.3% in all severe hospitalised patients, 23.7% in patients admitted to the ICU and 17.2% in patients who died), as was also observed in the study by Xu et al. [Reference Xu21] (34.6% in moderately ill patients, 17.5% patients admitted to the ICU and 14.3% in patients who died).
Patients aged ⩾75 years received NAI less frequently (87.8%) than those aged 18–64 years (93.2%) in our study, in contrast to the study by Rolfes et al. [Reference Rolfes22] in a Connecticut (USA) tertiary hospital, in which adults aged ⩾75 years were more frequently prescribed NAI than younger patients during the 2010–2011 to 2012–13 seasons. In the study by Lindegren et al. in four US hospitals, carried out from 2006 to 2012 in laboratory-confirmed cases, NAI treatment was more common in patients aged ⩾65 years than in those aged 50–64 years, although the differences were not statistically significant [Reference Lindegren6].
In agreement with other authors [Reference Lindegren6, Reference Rolfes22], we found no association between comorbidities and receiving NAI treatment. In contrast, Appiah et al. [Reference Appiah17] in an all-ages study found that patients with comorbidities more frequently received antivirals than those without.
We found that a higher proportion of influenza A cases received NAI than influenza B cases (92.8% vs. 82.2%, P < 0.001). In the 2010–2011 Spanish study by Gutiérrez-Pizarraya et al. patients with confirmed influenza A virus who presented primary pneumonia had received NAI more frequently that those with the influenza B virus [Reference Gutiérrez-Pizarraya23]. In the Canadian study by McGeer et al., NAI treatment was also more frequently administered to influenza A patients [Reference McGeer8]. This may be because clinicians know that excess mortality is higher when one of the virus A subtypes (H3N2 subtype) predominates [Reference Treanor, Bennet, Dolin and Blaser3].
The multivariable analysis showed that NAI were effective in avoiding death in hospitalised patients when administered within the 48 h following symptom onset (aOR 0.37, 95% CI 0.22–0.63) and less effective in avoiding death when administered ⩾48 h after symptom onset (aOR 0.62, 95% CI 0.40–0.97) (Table 4). This is in agreement with other studies. Hiba et al. in Israel in 2009–2010 compared patients hospitalised due to influenza who received NAI ⩽48 and >48 h after symptom onset and found that mortality was higher in patients with delayed treatment [Reference Hiba24]. In a Hong Kong study by Lee et al. [Reference Lee25], carried out between January 2007 and December 2008, early NAI treatment (⩽48 h) was associated with better survival. In Spain, the study by Delgado-Rodríguez et al. [Reference Delgado-Rodríguez26] in 2009–2010 found that NAI were only effective in avoiding death or ICU admission when administered ⩽48 h, with an aOR of 0.46 (95% CI 0.27–0.80), close to that obtained in our study.
A meta-analysis of subjects included in observational studies concluded that, compared with no treatment, NAI administered at any time were associated with a reduced risk of mortality (OR 0.81, 95% CI 0.70–0.93), and for early treatment (⩽48 h) the OR was 0.50 (95% CI 0.37–0.67) [Reference Muthuri27].
In our study, the aOR of early NAI treatment (⩽48 h after symptom onset) in avoiding death in patients admitted to the ICU was 0.32 (95% CI 0.14–0.74) (Table 4), lower than the 0.44 (95% CI 0.21–0.87) obtained in another Spanish study carried out in 2010–2011 [Reference Rodríguez28]. In the US study by Shah et al. [Reference Shah20] in ICU patients in 2013–14, adult age was associated with death, as in the present study, but NAI administered ⩽48 h were not associated with survival.
The Hong Kong study by Lee et al. found that higher viral loads correlated with more severe symptoms. They pointed out that, in high-risk patients with severe symptoms, the viral load may remain high for longer, and therefore late initiation of NAI treatment may still be worth considering [Reference Lee29].
One important finding of the present study is that death was avoided not only in all severe hospitalised patients and patients admitted to the ICU who received NAI in the first 48 h after symptom onset but also, although to a lesser extent, in patients who received NAI within 3 days after symptom onset and 5 days after symptom onset. In the study by Louie et al., critically ill patients with A(H1N1) pdm09 who received NAI within 5 days after symptom onset had better survival than patients who did not receive NAI [Reference Louie30]. As McGeer et al. [Reference McGeer8] point out, these findings do not contradict data from healthy adult studies demonstrating that treatment should be started sooner than 48 h after symptom onset in order to reduce the duration of symptoms and severity of illness [Reference Treanor, Bennet, Dolin and Blaser3, Reference Yu31]. In the present study, most patients presented comorbidities and because, in immunocompromised patients, viral replication lasts longer than in healthy patients, in many of these patients, treatment might have had an effect on the progression of the disease and on avoiding death (see Supplementary Table S3).
Our results also suggest a certain dose–response relationship between the effectiveness of NAI in avoiding death and the days from symptom onset to the initiation of treatment. These results are in accordance with the findings of Louie et al. [Reference Louie30], who observed a trend toward improved survival in patients receiving the earliest treatment.
This study, like all observational studies, has strengths and limitations. One strength is that few studies have investigated the effectiveness of NAI in adult hospitalised patients, and therefore, our results may help identify factors associated with the suboptimal use of antiviral treatment in diminishing the burden of influenza disease in adults, while an RCT that could demonstrate causality would be difficult for ethical reasons [Reference Rodríguez28]. A second strength is that all cases included were laboratory-confirmed, and accordingly, we were able to investigate the effect of antiviral treatment in avoiding death due to influenza virus. Finally, the sample size permitted a multivariable analysis and therefore reduced the possibility of confounding factors invalidating the results.
The study has also limitations. First, hospitals participated voluntarily, which could lead to selection bias [Reference Xu21, Reference Rodríguez28, Reference Louie30, Reference Ayscue32]. However, because the hospitals participating in the surveillance system cover more than 60% of the population of Catalonia and we used a mixed-effect logistic regression model with hospitals as a random intercept, we believe that our results may be extensible to severe hospitalised patients in Catalonia. Second, unmeasured confounders, such as the characteristics of unmeasured comorbidities, might have altered the results. Third, untreated patients may have been less severely ill than patients treated with NAI, because the decision to treat with NAI may have been influenced by clinicians’ perceptions of disease severity [Reference Louie30]. The final adjusted model was constructed taking into account the propensity score built with variables that included the most important comorbidities, and it seems unlikely that the results suffered a large bias. Nevertheless, some residual confounding cannot be ruled out. Finally, because the subjects studied were severe hospitalised patients, the results cannot be extrapolated to outpatients or non-severe patients.
In conclusion, our results show that NAI treatment had a protective effect in avoiding death in patients hospitalised due to severe influenza and those admitted to the ICU. The effect was greater when administered ⩽48 h after symptom onset but also when no more than 5 days since symptom onset had elapsed.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000663
Acknowledgement
The Surveillance of Hospitalized Cases of Severe Influenza in Catalonia Working Group is composed of: Alsedà M, Álvarez J, Arias C, Balañà PJ, Barrabeig I, Camps N, Carol M, Ferràs J, Ferrús G, Follia N, Godoy P, Bach P, Jané M, Martínez A, Minguell S, Parrón I, Plasència E, Sala-Farré MR, Torner N, Torra R, Torres J (Public Health Agency of Catalonia); Caylà J, Gorrindo P, Rius C (Public Health Agency of Barcelona); Marcos MA, Mosquera MDM, Vilella A (H Clínic, Barcelona); Antón A, Pumarola T, Campins M (H Universitari Vall d'Hebrón, Barcelona); García D (H Josep Trueta, Girona); Espejo E (H Terrassa); Freixas N, Riera Garcia M (Mútua Terrassa); Maraver E, Mas D, Perez R, (H Altahia Manresa); Rebull J (H.Verge de la Cinta, Tortosa); Pou J (H Sant Joan de Déu, Esplugues); García-Pardo G, Olona M (H Joan XXIII, Tarragona); Barcenilla F, Castellana D (H Arnau de Vilanova, Lleida) Navarro-Rubio G (Consorci Sanitari Parc Taulí, Sabadell); Force LL (H Mataró).
Financial support
This study was supported by the Programme of Surveillance, Prevention and Control of Transmissible Diseases (PREVICET) of CIBER de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III, Madrid.
Conflict of interest
None.







