INTRODUCTION
Escherichia coli O157:H7 is a significant cause of enteric illness, resulting in an estimated 96 000 infections and 31 deaths each year in the USA [Reference Scallan1]. E. coli O157:H7 infections are primarily of foodborne origin, but infections also occur through contact with contaminated recreational water and direct contact with an infected animal or human [Reference Scallan1–Reference Kassenborg4]. Cattle and other ruminants are the predominant reservoir for E. coli O157:H7, and beef products, particularly ground beef, have been associated with outbreaks and sporadic cases [Reference Rangel3–Reference Swanson-Laine5]. However, fresh produce and other food items have also been associated with outbreaks [Reference Rangel3, Reference Lynch, Tauxe and Hedberg6–Reference Ferguson8].
The development of molecular subtyping by pulsed-field gel electrophoresis (PFGE) has revolutionized E. coli O157:H7 surveillance [Reference Tauxe9, Reference Gerner-Smidt10]. PulseNet, the national molecular subtyping network for foodborne disease surveillance, provides a standardized method for subtyping of E. coli O157:H7 isolates and normalizing PFGE patterns against a global reference standard provided by the CDC [Reference Swaminathan11, Reference Hunter12]. Molecular subtyping enhances case definition specificity, enabling outbreaks to be detected and controlled at an earlier stage, and allowing for the detection of geographically dispersed outbreaks [Reference Tauxe9, Reference Gerner-Smidt10, Reference Allos13–Reference Barrett15].
While the benefits of molecular subtyping, particularly by PFGE, in foodborne disease outbreak detection and investigation have been well established, there is no consensus about when an E. coli O157:H7 PFGE cluster warrants further investigation and almost no quantitative analysis about characteristics of PFGE clusters that indicate a common source will be identified [Reference Gerner-Smidt10, Reference Buehler16–20]. Cluster size and the number of days from receipt of the first cluster case isolate to the third case isolate by the public health laboratory were found to be significant predictors of a source of infection being identified for Listeria monocytogenes clusters in France and Salmonella clusters in Minnesota [Reference Hedberg, Jacquet and Goulet21, Reference Rounds22]. The purpose of this study was to determine the characteristics of E. coli O157:H7 clusters identified by the Minnesota Department of Health (MDH) that could serve as useful predictors of their being solved (i.e. result in identification of a confirmed outbreak).
METHODS
E. coli O157:H7 infections are reportable to MDH by state rule [23]. Clinical laboratories are required to forward all E. coli O157:H7 isolates to the MDH Public Health Laboratory (PHL). PFGE subtyping after digestion with XbaI is conducted according to PulseNet protocols on all isolates as soon as they are received [Reference Ribot24]. Prior to 2006, additional PFGE subtyping after digestion with BlnI was only conducted on isolates when requested by MDH epidemiologists. Beginning in 2006, PFGE subtyping after digestion with BlnI was routinely conducted on all isolates. PFGE subtypes are uploaded to the national PulseNet database [Reference Ribot24].
All Minnesota residents with a culture-confirmed E. coli O157:H7 infection are routinely interviewed by MDH staff with a standard questionnaire about symptom history, food consumption, water exposures, animal contact, daycare, and other potential exposures occurring in the 7 days before onset of illness [Reference Swanson-Laine5, Reference Smith25]. The questionnaire contains detailed food exposure questions, including open-ended food histories and objective yes/no questions about numerous specific food items, as well as brand names and purchase locations. As with Salmonella, E. coli O157:H7 clusters are investigated using a dynamic model in which suspicious exposures identified during initial case interviews are added to the standard interview for subsequent cases [Reference Rounds22, Reference Smith25]. Similarly, initial cases may be re-interviewed to ensure uniform ascertainment of the suspicious exposures. This iterative approach is used to identify exposures for further evaluation with formal hypothesis testing, product sampling, and/or product tracing [Reference Rounds22, Reference Smith25].
A cluster was defined as ⩾2 cases of E. coli O157:H7 in different households with isolates of the same XbaI PFGE subtype and with specimen collection dates within 2 weeks [Reference Rounds22, Reference Bender26]. Thus, a single cluster would be ongoing as long as a new isolate was collected within 2 weeks after the most recent isolate in the cluster. A cluster was considered solved if the epidemiological evaluation of that cluster resulted in the identification of a common source of infection for those cases and consequently the documentation of a confirmed outbreak.
Inclusion and exclusion criteria
Laboratory-confirmed cases of E. coli O157:H7 infection in Minnesota residents with specimen collection dates from 1 January 2000 to 31 December 2008, for which isolates were received and subtyped by MDH PHL, were included in the study. Isolates not received through routine surveillance (i.e. testing was requested or conducted by MDH as a part of an ongoing investigation) were excluded from the analysis.
Solved clusters were included if they were detected and solved solely on the basis of investigation of cases identified through submission of isolates to MDH through routine laboratory surveillance. Solved clusters in which a call to the MDH foodborne illness complaint hotline (e.g. from the public) or a report of a cluster of illnesses from a healthcare provider or institution directly contributed to the identification of an outbreak were excluded from analysis [Reference Li27]. Secondary clusters, defined as clusters in which the cases were part of a confirmed outbreak that had been previously identified, were also excluded from the analysis.
Study variables
Variables incorporated into the analysis included cluster size and cluster case density. Cluster size was defined as the number of cases in each cluster and was categorized into cluster sizes of 2, 3, 4, and ⩾5. For clusters in which a common source was identified, only cases that were received before the cluster was solved were included. Cluster case density was defined as the number of days from receipt date of the first cluster isolate at MDH PHL to the receipt date of the second cluster isolate and was categorized into cluster case densities of 0 days (i.e. isolates were received on the same day), 1–7 days, and ⩾8 days.
The relationship between common and uncommon PFGE subtypes and solving a cluster was examined. Clusters with PFGE subtypes representing ⩾2% of all E. coli O157 isolates received at MDH PHL during 2000–2008 (CDC PFGE subtype designations EXHX01.0047, EXHX01.1343, EXHX01.0074, EXHX01.0087, EXHX01.0008, EXHX01.0224, EXHX01.0238) were categorized as common, and all other subtypes were categorized as uncommon.
The relationship between seasonality and solving a cluster was examined. Clusters in which the first cluster isolate was received at MDH PHL from June to September were categorized as occurring during peak season. Clusters in which the first cluster isolate was received from October to May were categorized as occurring during low season.
Outbreaks were classified as being detected through the investigation of a PFGE cluster, the routine follow-up of a case due to reported child daycare exposure or long-term care facility exposure, a report of a cluster of illnesses from a healthcare provider or institution, or a consumer call to the foodborne illness complaint hotline. The number of days from the illness onset of the first outbreak case until the outbreak was solved was calculated for each outbreak. Outbreaks were also classified by route of transmission, including foodborne, person-to-person, animal contact, and waterborne transmission.
Outbreaks involving one facility (restaurant, child daycare centre, school) or event were classified as point source. Outbreaks involving commercially distributed food items at multiple points of sale (grocery stores, restaurants) were classified as non-point source. The time required to interview each E. coli O157:H7 case with the MDH standard questionnaire was recorded for a 6-month period in 2008, and the median interview time was calculated.
Analyses
Descriptive analyses were conducted to characterize outbreaks with respect to route of transmission and method of detection. A descriptive analysis was also conducted to characterize the frequency of E. coli O157:H7 PFGE subtypes. The Mantel–Haenszel χ2 test for trend was used to characterize temporal trends in the number of clusters that were solved. Two-sided Wilcoxon rank-sum tests were used to compare the median cluster size and cluster case density of point-source and non-point-source outbreaks. Two-sided Wilcoxon rank-sum tests were used to compare the number of days from the illness onset of the first outbreak case until the outbreak was solved by method of outbreak detection. Univariate analyses were performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) characterizing the crude associations between E. coli O157:H7 cluster PFGE subtype, cluster size, cluster case density, and a cluster being solved. The effect of the use of a second restriction enzyme (BlnI) was measured descriptively and by univariate analysis comparing the percentage of clusters solved from 2000 to 2005 to the percentage solved from 2006 to 2008. A logistic regression model was used to estimate associations between variables that were significant in the univariate analysis. Mantel–Haenszel χ2 tests for trend and interaction terms were used to investigate the linear nature of the relationship between both cluster size and cluster case density and the outcome. SAS software version 9.1 (SAS Institute, USA) was used for descriptive analyses, univariate analyses, and logistic analyses. An alpha of ⩽0·05 was considered significant.
RESULTS
During 2000–2008, a total of 1181 clinical E. coli O157:H7 isolates from Minnesota residents were received at MDH through routine surveillance; these represented 89% of reported E. coli O157:H7 cases (n=1325, incidence 2·87 cases/100 000 person-years). PFGE subtyping was performed on all 1181 isolates, and all were included in the study. Of these isolates, 575 E. coli O157:H7 PFGE subtypes were observed. The seven most common subtypes were (PulseNet designations) EXHX01.0047, 74 (6%) isolates; EXHX01.1343, 50 (4%) isolates; EXHX01.0074, 37 (3%) isolates; EXHX01.0087, 31 (3%) isolates; EXHX01.0008, 22 (2% isolates); EXHX01.0224, 20 (2%) isolates; and EXHX01.0238, 19 (2%) isolates. The remaining 568 PFGE subtypes each accounted for <1% of all isolates.
Interviewing each case of E. coli O157:H7 required a median of 32 min using MDH's standard questionnaire. MDH staff spent approximately 70 h per year conducting routine interviews of E. coli O157:H7 cases. This figure does not include time spent attempting to reach cases, gathering demographic information from clinicians, or re-interviewing cases for cluster investigations.
Outbreak and cluster characteristics
During 2000–2008, a total of 40 confirmed E. coli O157:H7 outbreaks involving Minnesota cases were identified, representing 289 (22%) isolates. Eighteen (45%) outbreaks were due to foodborne transmission, 15 (37·5%) were due to person-to-person transmission, five (12·5%) were due to animal contact, and two (5%) were due to waterborne transmission (Table 1).
Table 1. Outbreaks of E. coli O157:H7 infection by route of transmission and method of detection, Minnesota, 2000–2008
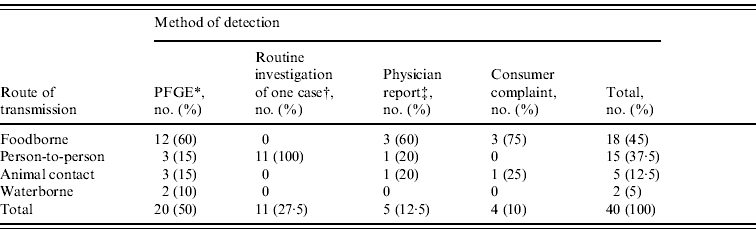
* Pulsed-field gel electrophoresis subtyping of isolates submitted as part of routine laboratory-based surveillance.
† Routine investigation of one case due to reported child daycare exposure or long-term care facility exposure revealed additional illnesses.
‡ A report of multiple illnesses from a healthcare provider or institution.
Twenty-one (53%) of the 40 outbreaks were excluded from the cluster analysis. Eleven outbreaks, occurring in child daycare centres (n=10) and a long-term care facility (n=1), were identified through the routine follow-up of only one case. In five outbreaks, a report of multiple illnesses from a healthcare provider or institution led to the identification of the outbreak. In four outbreaks, a consumer illness complaint contributed to the identification of the outbreak. One outbreak was detected through PFGE subtyping but had isolates that did not meet the cluster definition. The remaining 19 outbreaks, representing 175 (13%) isolates, were included in the analysis and comprised 12 (63%) foodborne, three (16%) person-to-person, two (10·5%) animal contact, and two (10·5%) waterborne outbreaks.
Compared to outbreaks detected through PFGE subtype surveillance alone, the time interval from the first outbreak case's illness onset to the date the outbreak was solved was significantly shorter for outbreaks detected through a report of multiple illnesses from a healthcare provider or institution, and for outbreaks detected through the routine follow-up of only one case due to child daycare or long-term care facility exposure. In addition, the difference in this interval between outbreaks detected by PFGE subtyping and outbreaks detected through consumer complaints approached statistical significance (Table 2).
Table 2. Timeliness of E. coli O157:H7 outbreak investigation by method of detection, Minnesota, 2000–2008
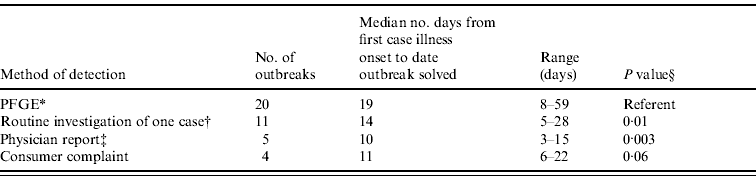
* Pulsed-field gel electrophoresis subtyping of isolates submitted as part of routine laboratory-based surveillance.
† Routine investigation of one case due to reported child daycare exposure or long-term care facility exposure revealed additional illnesses.
‡ A report of multiple illnesses from a healthcare provider or institution.
§ Wilcoxon rank-sum test comparing median number of days from first case illness onset to date outbreak solved for outbreaks detected through PFGE subtype surveillance alone with outbreaks detected through other methods.
Of the 19 outbreaks detected through PFGE subtyping alone, nine (47%) involved one facility, location or event and therefore were classified as point source. The other 10 (53%) outbreaks involved commercially distributed food items at multiple points of sale (grocery stores, restaurants) and therefore were classified as non-point source. The median cluster size of point-source outbreaks was four cases, and the median cluster size of non-point-source outbreaks was three cases (P=0·78). The median cluster case density was 4 days for point-source outbreaks and 1·5 days for non-point-source outbreaks (P=0·84).
A total of 100 E. coli O157:H7 PFGE clusters were detected in the study period, representing 374 (32%) isolates. Sixteen (16%) clusters were excluded from the analysis: seven clusters in which a report of multiple illnesses from a healthcare provider or the public directly contributed to the identification of the outbreak; five clusters in which the routine follow-up of the first cluster case led to the identification of the outbreak; and four secondary clusters). Nineteen (23%) of the 84 clusters included in the analysis were solved (i.e. they led to the documentation of a confirmed outbreak).
Temporal trends, seasonal trends, and cluster PFGE subtype
The median number of E. coli O157:H7 isolates subtyped per year was 129 (range 83–196). The median number of E. coli O157:H7 clusters per year was 11 (range 4–18). The median number of confirmed E. coli O157:H7 outbreaks per year was five (range 3–6). There were no significant trends in the proportion of E. coli O157:H7 clusters that resulted in the identification of a confirmed outbreak (P=0·25) (Fig. 1).
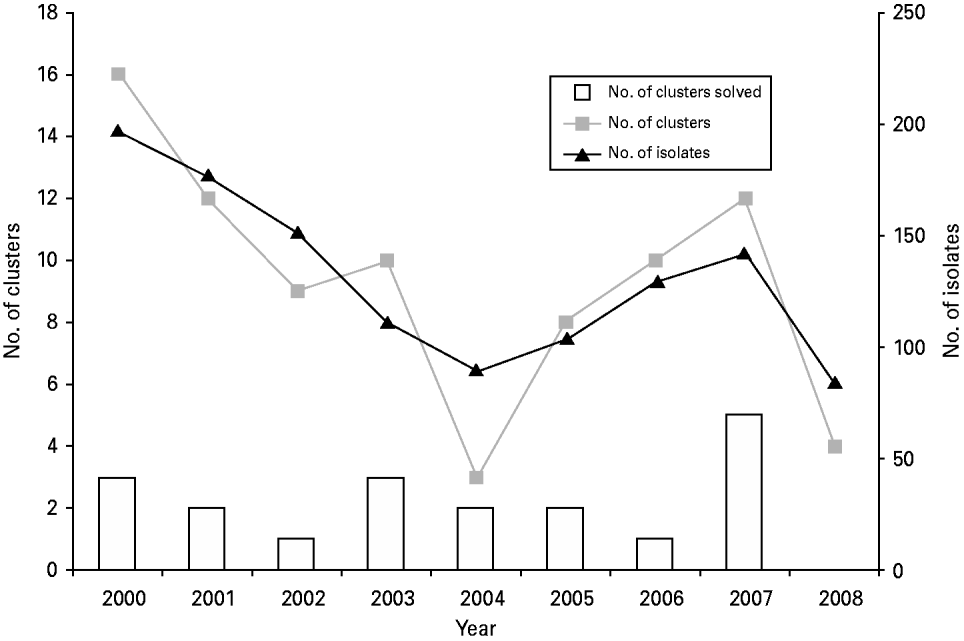
Fig. 1. Temporal trends in number of clinical E. coli O157:H7 isolates received, number of pulsed-field gel electrophoresis clusters, and number of clusters solved, Minnesota, 2000–2008.
The number of isolates, clusters, and solved clusters exhibited seasonal variation. Sixty-two percent of all isolates, 71% of all clusters, and 79% of all solved clusters occurred during the 4-month period of June to September (Fig. 2). However, low season clusters were not significantly more likely to be solved than peak season clusters (OR 1·7, 95% CI 0·5–7·7).
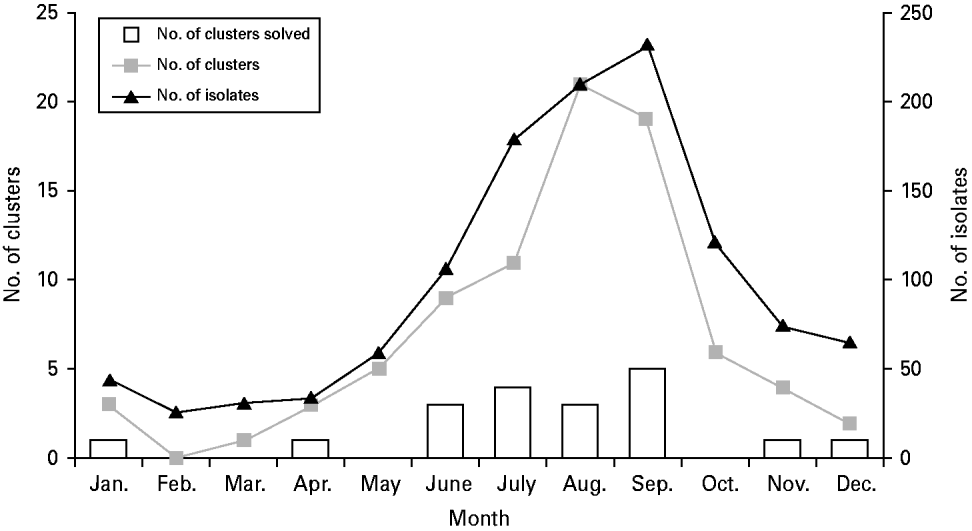
Fig. 2. Seasonal distribution of number of clinical E. coli O157:H7 isolates received, number of pulsed-field gel electrophoresis clusters, and number of clusters solved, Minnesota, 2000–2008.
Uncommon PFGE subtype clusters were not significantly more likely to be solved than common PFGE subtype clusters (OR 1·1, 95% CI 0·4–3·3) (Table 3).
Table 3. Univariate association between E. coli O157:H7 pulsed-field gel electrophoresis subtype frequency, cluster size, cluster density and cluster being solved, Minnesota, 2000–2008
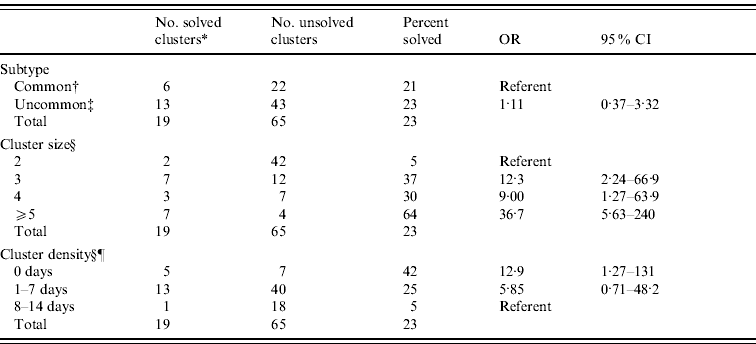
* A cluster was considered solved if the epidemiological evaluation of that cluster resulted in the identification of a common source of infection for those cases and consequently the documentation of a confirmed outbreak.
† PulseNet subtype designations EXHX01.0047, EXHX01.1343, EXHX01.0074, EXHX01.0087, EXHX01.0008, EXHX01.0224, and EXHX01·0238; each represented ⩾2% of all E. coli O157:H7 isolates.
‡ All other subtypes.
§ Significant Mantel–Haenszel χ2 test for trend P<0·05.
¶ Cluster density measured as the number of days from receipt of first cluster case isolate to receipt of second case isolate at the Minnesota Department of Health Public Health Laboratory.
Cluster size
The probability of a cluster being solved increased significantly as the number of cluster cases increased (Mantel–Haenszel χ2 test for trend 21·2, P<0·001) (Table 3). Sixty-four percent of clusters of ⩾5 cases were solved compared to 37% of clusters of four cases, 30% of clusters of three cases, and 5% of clusters of two cases. The odds of solving a cluster of ⩾5 cases were 36·7 times higher than the odds of solving a cluster of two cases. Clusters of four cases were 9·0 times more likely to be solved than clusters of two cases. Clusters of three cases were 12·3 times more likely to be solved than clusters of two cases (Table 3). There was statistical evidence of a nonlinear relationship between cluster size and solving the cluster (Wald χ2 for interaction 8·4, P<0·004).
Cluster case density
The proportion of clusters that were solved increased significantly as the density of cluster cases increased (Mantel–Haenszel χ2 test for trend 5·79, P=0·016) (Table 3). The odds of solving a cluster if the first two case isolates were received on the same day were 12·9 times higher than the odds of solving a cluster in which the first two case isolates were received within 8–14 days. Clusters in which the first two case isolates were receive within 1–7 days were 5·9 times more likely to be solved than clusters in which the first two case isolates were received over 8–14 days, but the difference was not statistically significant (Table 3).
Use of a second PFGE restriction enzyme
The number of isolates and clusters for which a second restriction enzyme (BlnI) was used increased over the study period until it was routinely performed on all E. coli O157:H7 isolates received by MDH PHL beginning in 2006. Applying BlnI results to the XbaI PFGE cluster definition for the 31 XbaI PFGE clusters identified from 2006 to 2008 eliminated five clusters of two isolates each and removed four isolates from other clusters. Incorporating the BlnI results, clusters from 2006 to 2008 were not significantly more likely to be solved than clusters from 2000 to 2005 (OR 1·3, 95% CI 0·4–4·2, P=0·66).
Logistic regression
The logistic regression model included cluster size and cluster case density. Only cluster size remained associated with solving a cluster in the final model. The adjusted odds of a cluster being solved were 2·2 times higher (95% CI 1·4–3·6) for each additional cluster case.
DISCUSSION
During the study period, 84 E. coli O157:H7 PFGE clusters were identified and 19 (23%) were solved. Cluster size was the most useful predictor of a cluster being solved; there was a significant increase in the success of solving clusters of ⩾3 cases. The proportion of clusters that were solved increased as the number of cases in the cluster increased, up to ⩾5 cases. The observed association is logical because as the number of cluster cases increases, the amount of epidemiological data available for evaluation also increases. Moreover, larger clusters increase the likelihood that the cluster cases are epidemiologically linked rather than unrelated sporadic cases.
Cluster PFGE subtype frequency was not found to be a predictor of a cluster being solved in this study. In theory, isolates with uncommon PFGE subtypes are more likely to be epidemiologically linked compared to isolates with a common PFGE subtype, which sometimes represent unrelated sporadic cases that have a temporal association by chance. There are several possible reasons that no association was found. There were a relatively small number of clusters and solved clusters included in this study, and this sample size may not have been large enough to detect a true association. There is also no definitive definition of what should be considered common or rare PFGE subtypes or where this division should be. In this study, a natural break in the frequencies of PFGE subtype was used. The results of this study suggest that PFGE subtype frequency should not be used in deciding whether to investigate an E. coli O157:H7 PFGE subtype cluster at the local level.
We strongly recommend interviewing all E. coli O157:H7 cases and investigating all PFGE subtype clusters to identify as many outbreaks as possible. Interviewing cases after a cluster has been identified may result in poorer case exposure recall, affecting the ability to detect an outbreak's source and delaying interventions. The large proportion of outbreaks that were not detected through the investigation of PFGE clusters alone, particularly the 10 outbreaks detected in childcare settings through the routine interview and follow-up of only one case, demonstrates the considerable public health benefit in routinely interviewing all E. coli O157:H7 cases. However, if public health resources absolutely do not permit conducting routine interviews of all E. coli O157:H7 cases and investigating all PFGE clusters, then PFGE subtype clusters of ⩾3 E. coli O157:H7 cases should be investigated.
One confirmed outbreak during the study period did not have isolates that met the cluster definition and many confirmed outbreaks had cases that were outside the cluster definition. This finding is an important reminder that lack of temporal clustering in a 2-week time-frame does not eliminate the possibility of an outbreak. In four outbreaks a call placed to MDH's foodborne illness hotline contributed to the outbreak's detection and identification of the source, demonstrating the utility of complaint systems in detecting some outbreaks [Reference Li27].
Outbreaks detected through the report of multiple illnesses from a healthcare provider or institution, the routine interview and follow-up of one case reporting daycare or long-term care facility exposure, or through consumer complaints were solved more quickly than outbreaks detected through the investigation of PFGE clusters alone, demonstrating the utility of these surveillance methods. A comprehensive surveillance system integrating real-time PFGE subtyping of all isolates, interviewing all cases in real-time using a detailed exposure questionnaire, and an organized system to field and process consumer complaints and physician reports allows for the optimal detection and control of outbreaks caused by E. coli O157:H7.
The results of this study are based on the characteristics of the population of Minnesota and MDH surveillance methods: conducting real-time PFGE subtyping of all E. coli O157:H7 isolates, interviewing all cases in real time using a detailed exposure questionnaire from a central location for the entire state, and aggressively investigating all clusters using an iterative model. These factors aid in the sensitivity and timeliness of outbreak detection and investigation in Minnesota. Additional studies in other states and at the national level are needed to understand surveillance characteristics in other states and determine useful predictors of multi-state clusters being solved.
A potential limitation of this study is the changing use of a second PFGE restriction enzyme over the study period. Starting in 2006, the routine use of a second restriction enzyme, BlnI, improved cluster investigation specificity by removing one isolate from four clusters and eliminating five clusters of two isolates. However, there was no difference in the proportion of clusters solved when BlnI was routinely used compared to when it was not routinely used. Therefore, contrary to a conclusion in a previous report, our data suggest that while the use of a second PFGE restriction enzyme improves case definition specificity and therefore has value, investigation of clusters should not be delayed until PFGE results from a second enzyme digestion are available [Reference Lynch18, Reference Gupta28, Reference Johnson29].
In summary, this study demonstrates the increased probability of an E. coli O157:H7 PFGE subtype cluster being solved as the number of cases in a cluster increases. Specifically, investigation of PFGE clusters of ⩾3 E. coli O157:H7 case isolates yielded a substantial benefit in terms of outbreak identification.
ACKNOWLEDGEMENTS
We thank the Public Health Laboratory staff and Foodborne Disease Unit staff at the Minnesota Department of Health. This work was supported in part through cooperative agreements with the Centers for Disease Control and Prevention (CDC) Emerging Infections Program, Foodborne Diseases Active Surveillance Network (FoodNet) (U50/CCU511190-10) and the CDC Epidemiology and Laboratory Capacity for Infectious Diseases Program (U50/CCU519683-04-4).
DECLARATION OF INTEREST
None.







