Psittacosis is a zoonotic infection, caused by the intracellular bacterium Chlamydia psittaci. It is best known for having its reservoir in ornamental pet birds (‘parrot fever’) but has been found in many other animal species, including in pigeons and poultry [Reference Heddema1, Reference Lagae2]. Symptoms are often of respiratory nature (pneumonia), but the infection may also remain asymptomatic, present as nonspecific febrile illness or as invasive disease such as meningitis, hepatitis or sepsis [Reference Heddema3, Reference Yung and Grayson4]. Awareness of psittacosis is generally low, both among clinicians and the general public [Reference Beeckman and Vanrompay5]. Combined with the fact that C. psittaci is often not included in routine diagnostic panels, underdiagnosis of psittacosis is likely to occur [Reference Spoorenberg6] and the true public health importance of psittacosis is largely unknown.
Disease burden estimations combine occurrence and severity of a disease to estimate (and compare) health impact. Rankings of diseases by burden can be useful to aid policy prioritisation. Therefore, burden estimations are also able to shed more light on the public health importance of lesser-known diseases, such as psittacosis. The European Centre for Disease Prevention and Control (ECDC)-commissioned Burden of Communicable Diseases in Europe (BCoDE) project developed an incidence-based and pathogen-based methodology specifically to estimate the burden of infectious disease in disability-adjusted life years (DALY) [Reference Mangen7]. DALYs are the sum of years of life lost (YLL) due to mortality and the years of life lived with disability (YLD) which measures morbidity. The YLD is the product of incidence, duration and severity, the latter being defined by a ‘disability weight’ between 0 (perfect health) and 1 (death). The ECDC has developed a toolkit to enable countries to estimate and compare the burden of 38 infectious diseases by this methodology [8]. However, a model for psittacosis was not yet included. Using the BCoDE methodology, we developed a disease model for psittacosis to estimate its disease burden in the Netherlands, which can also be used by other countries.
We constructed an ‘outcome tree’ of the clinical courses of confirmed psittacosis patients based on a review of the literature (Fig. 1). We searched Medline for studies describing psittacosis cases or outbreaks, wherein descriptions and frequencies of symptoms were listed for laboratory-confirmed cases. We further asked experts if they knew about relevant data or publications for our purpose. Four studies were identified that described the clinical presentations of psittacosis infections [Reference Heddema3, Reference Yung and Grayson4, Reference Laroucau9, Reference Branley10]. To estimate the distribution of the distinct clinical features (‘health states’) within acute symptomatic infections, we performed a meta-analysis on data extracted from the literature using the package ‘metaprop’ in STATA version 13·0. Based on the results of our meta-analysis (Fig. S1), we assumed 5.6% of symptomatic infections to cause invasive illness (e.g. hepatitis, sepsis, meningitis); 45.0% to present as pneumonia and the remaining 49.4% to be a nonspecific febrile illness. No studies were found reporting a case fatality of psittacosis. Therefore, we performed a meta-analysis of two studies on outcomes of atypical pneumoniae in Dutch hospitalised patients (one for acute Q fever, one for atypical pneumonia), see Fig. S2 [Reference Kampschreur11, Reference Raeven12]. The result of this meta-analysis was a case fatality of 1.44% (95% CI 0.70–2.40%), which we modelled as β (10.9, 743) and multiplied by the proportion hospitalised psittacosis patients (modelled as β (202, 101)), to adjust for the fact that the studies contributing to the case fatality estimate were in hospitalised patients. This resulted in an overall case fatality estimate of 0.93% (95% CI 0.48–1.62%) We applied the case fatality estimate only to patients age 50 and older, as both studies found mortality mainly in adults above 50 years old [Reference Kampschreur11, Reference Raeven12]. We did not find enough evidence for more health state-specific case fatality estimates nor could we find evidence to justify further age- or sex-specific transition probabilities.
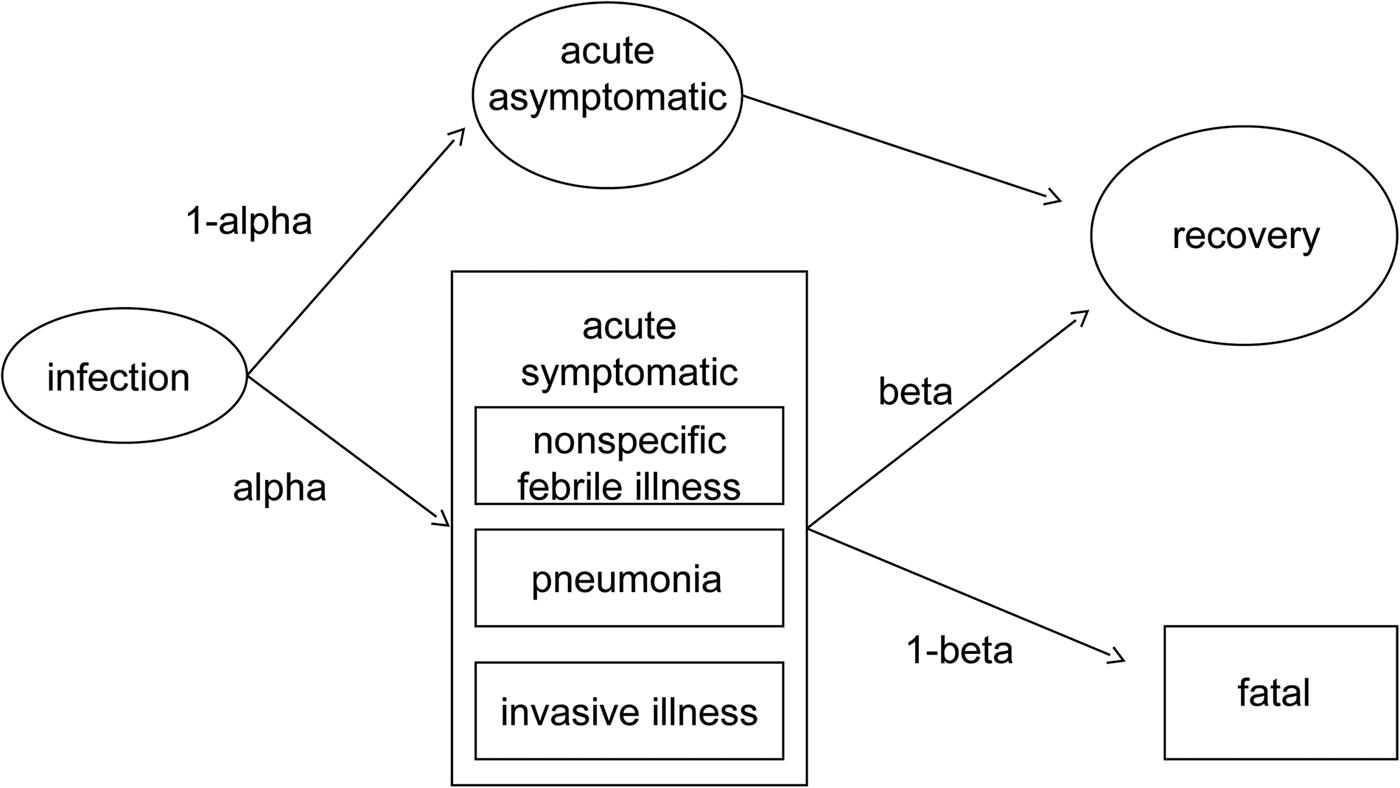
Fig. 1. Outcome tree of psittacosis disease progression model.
We applied disability weights recently estimated specifically for Europe [Reference Haagsma13]. Duration of pneumonia was set to 2 weeks, nonspecific febrile illness was assumed to last 5 days and invasive illness between 10 and 14 days (in accordance with the invasive pneumococcal disease in the BCoDE model). We assumed a disability weight of 0.051 (acute infectious disease episode, moderate) for nonspecific febrile illness [Reference Haagsma13]. For pneumonia, the disability weight of a severe acute infectious disease episode was assumed (0.125), as in the (healthcare-associated) pneumonia model. Invasive illness was given the disability weight of 0.655, defined for an intensive care unit admission, as was done for the invasive pneumococcal disease model by ECDC.
While psittacosis is a notifiable infectious disease in the Netherlands, we expect the notified cases to be an incomplete record of all psittacosis infections, due to underdiagnosis. To estimate a multiplication factor to apply to the notified cases to account for underestimation, we used the results of a systematic review and meta-analysis of the proportion of C. psittaci diagnoses in studies among community-acquired pneumonia (CAP) patients. This study is further described in reference [Reference Hogerwerf14]. In short, the result of this meta-analysis estimated 1.03% (95% CI 0.79–1.30%) C. psittaci infections in CAP patients. Table 1 shows the calculations by which we estimated the multiplication factor of 22.7 (95% CI: 12.2–64.6) to adjust for underestimation of the psittacosis incidence by national notifications. In other words, we estimate 4.4% (95% CI 1.6–8.2%) of symptomatic cases to be notified in the Netherlands. Calculations were performed in R [15] and uncertainty was propagated using Monte Carlo simulation; specifically 1 000 000 random draws were made from the defined distributions for each variable.
Table 1. Steps in the estimation of multiplication factor for underestimation of a total number of psittacosis infections in the Netherlands by national notification registry
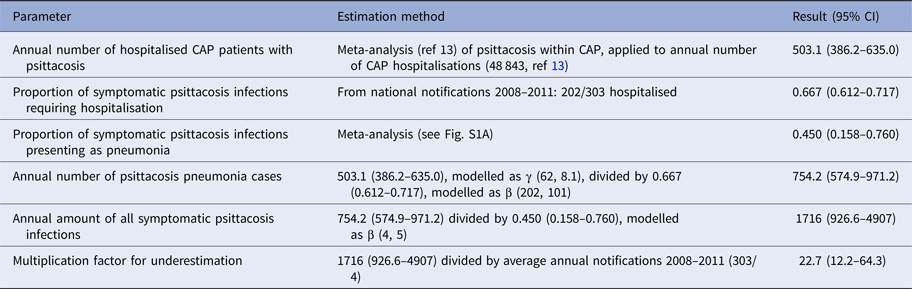
CAP, community-acquired pneumonia.
Average annual psittacosis notifications in 2012–2014 per age and sex were used as input. We ran the model in ECDC BCoDE toolkit version 1.2, with 10 000 iterations and no time discounting or age weighting. The model-based age- and sex-specific remaining life expectancies as in the 2010 Global Burden of Disease study was used and the average age distribution of the Dutch population in 2012–2014.
Our model estimated 1640 acute symptomatic cases of psittacosis and 9.7 deaths to have occurred annually in the Netherlands in 2012–2014. Men were more often affected than women, based on the notification data (61% vs. 39%). The estimated annual disease burden caused by psittacosis in the Netherlands was 222 DALY (95% CI 172–280). This corresponds to 1.32 (95% CI 1.02–1.67) DALY per 100.000 population. The disease burden was mainly due to mortality: 214 (95% CI 165–271) YLL and 7.6 (95% CI 6.5–8.7) YLD per year were estimated. The average DALY per case was 0.13 (95% CI 0.11–0.16). As mortality was only included in the model for patients above the age of 50, the disease burden was largely found in this group. Sixty-one per cent of notifications, concerned patients in this age category. When a time discount rate of 3% is applied, we estimate the psittacosis disease burden for 2012–2014 at 162 DALY/year (95% CI 128–205).
Aside from providing a first psittacosis disease burden estimation, this study has highlighted some evidence gaps. There is little information on clinical presentation of C. psittaci infection and one of four studies that we identified was published almost 30 years ago. However, as our review was not systematic, some published evidence may have been missed. We aimed to be conservative with our assumptions. However, the estimate of 67% hospitalisation of psittacosis cases may be too high. The evidence is lacking on mortality from psittacosis, although sporadic published reports and national notification data are in concordance with our estimate of around 1% mortality in older adults [Reference Petrovay and Balla16]. Since our disease burden estimate is mainly attributable to years of life lost, more evidence on psittacosis mortality is needed to better estimate disease burden. Further, long-term sequelae (such as fatigue) after acute psittacosis infection have not been described but may occur as with other (atypical) pneumoniae. A recent study showed a lower health-related quality of life in elderly up to 12 months after admission for community-acquired pneumonia compared with matched controls [Reference Mangen17]. Also, the large multiplication factor for underestimation highlights the uncertainty regarding the actual number of psittacosis patients in the Netherlands. Aside from surveillance or burden estimation purposes, underdiagnosis of this zoonosis is a serious issue as this may delay or even prevent appropriate therapy, as the prevailing professional guidelines recommend beta-lactam antibiotic therapy for the clinically diagnosed CAP, which is not effective against C. psittaci [Reference Spoorenberg6]. Also, undiagnosed psittacosis cases represent missed opportunities for source tracing and elimination to prevent further cases.
Our estimated psittacosis disease burden of 222 DALY/year is comparable with those of rubella (222 DALY/year) and shigellosis (179 DALY/year) during the same period (2012–2104), which are also undiscounted estimates [Reference Bijkerk18]. According to our estimations, more than 1500 symptomatic psittacosis patients remained undiagnosed yearly in the Netherlands in 2012–2014. These results warrant increased awareness among clinicians and the general public of this zoonosis and possibly prioritisation of policies aimed at reducing the psittacosis disease burden. With the model we constructed and the parameters reported here, other countries may assess their psittacosis disease burden as well. The model is hereby available in a format that can be adjusted and imported into the ECDC BCoDE toolkit (Supplementary file 1).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817003065.
Acknowledgements
We wish to acknowledge the valuable contributions of Barend Baan and Scott McDonald to this study, as well as constructive feedback from Marianne van der Sande and Jaap van Dissel (all RIVM). This study was funded by the Dutch Ministry of Health, Welfare and Sport and from the Plat4m-2Bt-psittacosis project, granted by ZonMw, the Netherlands Organization for Health Research and Development (project number 522001002).
Declaration of Interest
None.




