INTRODUCTION
Corynebacterium diphtheriae is the main causative agent of diphtheria, a toxemic disease whose prevention depends on the implementation of effective immunization programmes by using diphtheria toxoid (dT) vaccines [Reference Hadfield1]. Toxigenic C. diphtheriae may circulate in a community for 20 years after a reported case of diphtheria, even in countries where immunization programmes are followed with great efficiency [Reference Golaz2, Reference Wagner3]. The introduction of toxigenic strains in a susceptible population may result in diphtheria outbreaks. All these aspects emphasize the need of vaccination strategies directed at persons of all ages and different ethnic groups and continuous surveillance of a population's immunity and new diphtheria cases [Reference Dittman4]. Despite all relevant knowledge acquired in different areas (microbiology, pathology, internal and preventive medicine) during the years, eradication of C. diphtheriae has not yet been achieved [Reference Mattos Guaraldi, Hirata and Damasco5].
Diphtheria causes significant illness and death in developing countries where vaccination coverage is low. Higher risk of acquiring the disease and potentially life-threatening complications are possible in inadequately immunized or unimmunized travellers to countries with endemic diphtheria [Reference Mattos-Guaraldi6, Reference Bitragunta7].
More recently in the Americas, diphtheria outbreaks have occurred in Haiti and the Dominican Republic. Diphtheria is rare in the USA; the last case occurred in an elderly traveller returning from Haiti in 2003. Although diphtheria is uncommon in industrialized countries because of longstanding routine use of vaccines containing dT [8], a changing epidemiology has been observed: Corynebacterium ulcerans has emerged as an important diphtheria toxin-producing pathogen and in some countries the number of diphtheria cases due to this species exceed the number reported with the classical aetiological agent, C. diphtheriae. Despite this, its capacity to cause disease in humans, including the inhabitants of urban centres, is still often neglected [Reference Dias9].
In Brazil, a developing country presenting a very large territory ( 8 547 403·5 km2) with varied geographical, social and economic conditions unfavourable for prevention of infectious diseases, during the last decades cases of diphtheria have been notified in many states, including in Maranhão, a northern state [Reference Mattos-Guaraldi6, Reference Dias9–11]. Recently, one case of co-infection by C. diphtheriae and the infectious mononucleosis virus was also reported in a Brazilian 11-year-old child whose vaccination against diphtheria was incomplete [Reference Mattos-Guaraldi12]. It is important to mention that accurate data have not been available, particularly from the north, northern and central-west states, because reporting is infrequent, laboratory confirmation is not available, and the extent of carriers is not clearly known [Reference Mattos-Guaraldi6].
In view of these facts and considering that the vaccine does not affect the infection/colonization by C. diphtheriae, since it is only directed against the toxin, not the whole bacterium, the endemicity of the disease in Brazil motivates diagnostic procedures appropriate to the environment, i.e. epidemiological and molecular investigations of the microorganism. The aim of the present investigation was to describe microbiological, clinical and epidemiological aspects of a diphtheria outbreak that recently occurred in Maranhão, Brazil.
METHODS
Clinical and epidemiological features
Data provided by the Public Health Secretary of the state of Maranhão showed that from January to June 2010, suspect diphtheria cases (n = 57) and contacts of diphtheria patients (n = 117) were notified in different villages of three municipalities of Maranhão: Jatobá (n = 95), Colinas (n = 46) and São Domingos (n = 33).
Diphtheria cases were confirmed by laboratory, clinical or clinical-epidemiological criteria, accordingly to the recommendations laid out in the Epidemiological Surveillance Guide of the Brazilian Health Ministry [13].
Origin of diphtheria bacilli strains, culture conditions and phenotypic analysis
Microorganisms were isolated from clinical samples collected with swabs from the nasopharynx and throat of the individuals at the Central Laboratory of Public Health from the state of Maranhão (LACEN-MA) by methods described previously [Reference Funke, Bernard, Murray, Baron, Jorgensen, Landry and Pfaller14]. Gram-positive Corynebacterium-like colonies obtained from cultures of clinical specimens collected from six patients (case nos. 10–15) were sent to the Collaborating Centre for Reference and Research on Diphtheria/National Health Foundation/Ministry of Health – FNS/MS, Brazil (LDCIC/FCM/UERJ) for further phenotypic and genotypic analysis.
Positive bacterial cultures for irregular Gram-positive rods (IGPR) were preliminarily characterized by colonial morphology, pigmentation, haemolysis, and DNase activity. Phenotypic characterization of Corynebacterium-like colonies was performed by conventional biochemical assays and the semi-automatized API-Coryne System v. 3.0 (bioMérieux, France) with the API web decoding system (www.apiweb.biomerieux.com) [Reference Funke, Bernard, Murray, Baron, Jorgensen, Landry and Pfaller14–Reference Gomes16]. Toxigenicity was evaluated by Vero cell cytotoxicity assays as described previously [Reference Efstratiou17].
C. diphtheriae biovar mitis non-toxigenic ATCC 27 010 [C7 s(–) tox− (NCTC 11 397)] type strain and the homologous toxigenic ATCC 27 012 (tox+) strain from the American Type Culture Collection (USA), TR241 (sucrose fermenting strain) and the strain Park–Williams no. 8 (PW8), used for toxoid vaccine preparation were used as controls of experiments in addition to the C. diphtheriae biovar gravis VA01 strain and C. ulcerans 809 human isolate.
Stock cultures in 10% skim milk with 25% added glycerol were maintained at −70°C and recovered as required by cultivation in trypticase soy broth (TSB; Difco Laboratories, USA) [Reference Hirata18].
Antimicrobial susceptibility testing
The sensitivity to antimicrobial agents (Oxoid, UK), penicillin G (10 U), erythromycin (15 μg), clindamycin (2 μg), rifampicin (5 μg), tetracycline (30 μg), linezolid (30 μg) and vancomycin (30 μg) was determined by the disk diffusion method using inoculum equivalent to a 0·5 McFarland standard, according to Clinical Laboratory Standards Institute (CLSI) guidelines [19]. Plates were incubated at 37°C for 24 h and reconfirmed at 48 h using a cation-adjusted Mueller–Hinton agar with 5% sheep's blood. Breakpoints for the susceptible strains were used as suggested by CLSI. The breakpoints for Staphylococcus aureus established by CLSI were considered in cases of penicillin.
Genotypic identification and toxigenicity evaluation using the multiplex polymerase chain reaction (mPCR) technique
A mPCR using three different primer pairs developed for detection of C. diphtheriae and differentiation between toxigenic and non-toxigenic strains was performed based on protocols described elsewhere [Reference Pimenta20, Reference Pimenta21]: two primer pairs targeted to domains A and B of the tox gene (Dipht 2F and Dipht 4R, 719 bp) and a third primer pair targeted to a region of the dtxR gene (DtxR1F and DtxR1R, 258 bp) [Reference Pallen22, Reference Nakao23].
DNA fingerprinting by pulsed-field gel electrophoresis (PFGE)
Genomic DNA was prepared following a method described previously [Reference Baio24]. The DNA was cleaved with SfiI (New England BioLabs, USA) according to the manufacturer's instructions. PFGE was performed in 0·5 × Tris-borate-EDTA 1·2% agarose gels at 13°C with a CHEF DRII system (Bio-Rad, USA). The pulse times were 3–18 s over 20 h. A concatenated lambda DNA (New England BioLabs) was used as a molecular size marker. PFGE banding profiles were analysed using visual comparison among the strains and with the aid of automated analysis using the BioNumerics Fingerprinting software v. 4.0 (Applied Math, Belgium). PFGE types were identified by roman numerals and subtypes were identified by roman numerals followed by a letter. The similarity index of the strains was calculated using the Dice correlation coefficient with a band position tolerance of 1% and the unweighted pair-group method using average linkages (UPGMA) was used to construct a dendrogram. Strains were considered to belong to the same PFGE group if the similarity index was ⩾85% band-based similarity coefficients as the cut-off values.
RESULTS
Epidemiological features
Data provided by the Public Health Secretary of the state of Maranhão showed that from January to June of 2010, suspect diphtheria cases (n = 57) and contacts of diphtheria patients (n = 117) were notified in different villages of three municipalities of Maranhão: Jatobá (n = 95), Colinas (n = 46) and São Domingos (n = 33). A total of 27 diphtheria cases [(females (n = 18), males (n = 9)] was confirmed by laboratory (n = 9), clinical (n = 7) or clinical-epidemiological (n = 11) criteria (Table 1). Data displayed in Figure 1 showed that the majority of the confirmed cases occurred in Jatobá (n = 20). The highest number of diphtheria cases was observed from January to February. Most (n = 26) of the confirmed cases occurred in children and adolescents of varied ages [<7 years (n = 13), 7–15 years (n = 13)] that were partially (n = 16) or completely (n = 10) immunized. Only one case occurred in a 31-year-old and partially immunized female patient. Three cases ended up in death: one child was partially immunized, i.e. did not receive all doses of DTP vaccine provided in the Brazilian immunization schedule [13], and the other two were completely immunized.

Fig. 1. Diphtheria outbreak in the state of Maranhão, Brazil. From January to June 2010. Confirmed (n = 27) and suspect (n = 57) cases and contacts (n = 117) of diphtheria cases were notified in municipalities, located 400 km from the metropolitan area of São Luís (●), the capital of Maranhão State: (i) Jatobá with a surface area of 406 km2 and a population of 8526 inhabitants (95 diphtheria cases); (ii) Colinas with a surface area of 2034 km2 and a population of 39 167 inhabitants (46 diphtheria cases); and São Domingos with a surface area of 1303 km2 and a population of 33 630 inhabitants (33 diphtheria cases). † Fatal cases (n = 3).
Table 1. Clinical and epidemiological aspects of 27 confirmed cases of diphtheria notified during outbreak in Maranhão, Brazil (January–June, 2010)
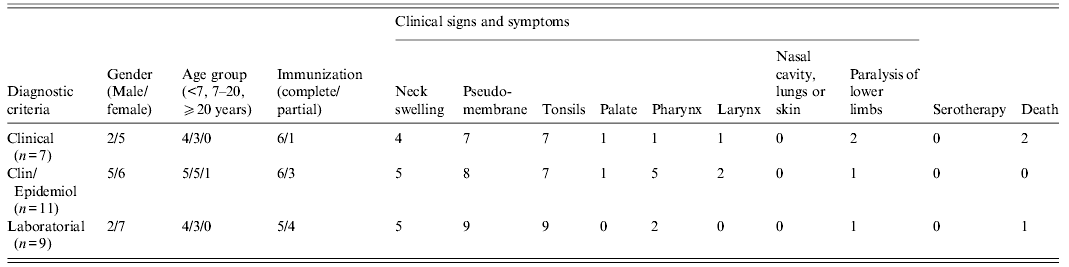
Clin/Epidemiol, Clinically compatible case epidemiologically linked to a laboratory-confirmed case; from 27 diphtheria cases, data of paralysis were not informed in nine patients: five and four cases with clinical and laboratory diagnosis, respectively.
Data were provided by the Health Secretary of the state of Maranhão.
Clinical signs and symptoms
Clinical signs and symptoms included fever, weakness, cervical lymphadenopathy (n = 14) and pseudomembrane formation in the tonsils (n = 25), palate (n = 2), pharynx (n = 8), larynx (n = 3) but not in the nasal cavity, lungs or skin; complications such as paralysis of lower limbs were also observed (n = 4). Most of the patients exhibited pseudomembranes (92·59%). Several patients (51·85%) also exhibited enlarged lymph nodes of the neck giving a ‘bull neck’ appearance. The patients were treated with antibiotics but were not submitted to anti-diphtheria serotherapy (Table 1).
C. diphtheriae phenotypic properties
All six strains presented phenotypic characteristics of C. diphtheriae biovar intermedius including non-haemolytic and lipophilic colonies <1 mm. Microorganisms were positive for catalase, DNAse, nitrate reductase and α-glucosidase. Pyrazinamidase, urease, gelatinase, alkaline phosphatase, esculin hydrolysis and CAMP tests gave negative results. Fermentation tests were positive for glucose, maltose, ribose and mannose; negative for glycogen, sucrose, xylose, mannitol, galactose, trehalose, arabinose and lactose. The API Coryne System confirmed the result, in which the samples showed a numerical profile 1 010 324.
Genotypic identification and toxigenicity evaluation by mPCR
Results of analysis by mPCR confirmed that all six strains tested corresponded to the species C. diphtheriae (dtxR gene positive). Similar to the (tox+) control C. diphtheriae ATCC 27012 strain, five strains exhibited the presence of the tox gene. An MA136 strain isolated from the throat with pseudomembrane of a child living in Colinas, gave negative results by mPCR for the tox gene analogous to the (tox−) control C. diphtheriae ATCC 27 010 strain. Complete agreement between the results of mPCR and the gold standard Vero cell cytotoxicity assays was observed for gene tox-positive C. diphtheriae strains tested.
Antimicrobial susceptibility profiles
All clinical isolates tested showed resistance to clindamycin and susceptibility to erythromycin, rifampicin, linezolid and vancomycin. Resistance to tetracycline was observed in three (MA23, MA131, MA150) strains. Two strains (MA23 and MA52) showed intermediate susceptibility to penicillin G.
PFGE analysis
Three distinct PFGE types (Ia, Ib, II) were found in the C. diphtheriae isolates from Maranhão (Fig. 2). The PFGE subtypes Ia and Ib showed a similarity coefficient ⩾95% and were considered genetically related. PFGE type Ia was the most frequently observed in the five strains evaluated in this study. Only the non-toxigenic MA136/13 strain exhibited PFGE type II. Three other PFGE types (III, IV, V) different from those presented by the strains from Maranhão were exhibited by the PW8 strain, which is the only major strain used in toxoid vaccine production and by TR241 and VA01 strains isolated from patients with diphtheria in Rio de Janeiro city [Reference Mattos-Guaraldi10].

Fig. 2. Pulsed-field gel electrophoresis (PFGE) types of Corynebacterium diphtheriae strains isolated from children with diphtheria living in the state of Maranhão, Brazil. Lane 1, λ DNA ladder PFGE marker; lanes 2–5, PFGE type Ia (MA19, MA23, MA52, MA131 strains, respectively); lane 6, PFGE type II (MA136 strain); lane 7, PFGE type Ib (MA150 strain); lane 8, profile III (TR241 strain); lane 9, profile IV (VA01 strain); lane 10, profile V (PW8 strain).
DISCUSSION
It is well known that the effectiveness of the vaccination using the dT against diphtheria caused by C. diphtheriae varies from 45% to 90% [Reference Phalkey25]. In the recent diphtheria outbreak in Maranhão the majority of Brazilian patients were children and many of them had been covered by a complete course of immunization. Cervical lymphadenopathy (70·37%) and neck oedema (51·85%) were observed in our study, indicating the occurrence of a severe form of the disease in Maranhão. The presence of pseudomembrane, a pathognomonic sign of the disease [Reference Hadfield1], was found in 92·59% of the patients despite immunization; mostly children (aged 1–12 years) and females; 11·12% cases were fatal, possibly due to the lack of diphtheria antitoxin and delayed treatment, as previously observed at the beginning of epidemic diphtheria in the states of the Former Union of Soviet Socialist Republics [Reference Dittman4]. In Dhule, India, there was a shift in the median age of disease to adolescents (10–15 years) without gender differences; only 18% reported disease despite immunization. About 64% of the confirmed cases presented with a well-defined pseudomembrane [Reference Phalkey25]. In the newly independent states of the Former Soviet Union, a high proportion of cases of pharyngeal or tonsillar diphtheria without pseudomembrane formation in adults were observed [Reference Kadirova, Kartoglu and Strebel26].
A case of diphtheria with pseudomembrane formation in a Brazilian 32-year-old woman has previously been reported. Her history included complete paediatric immunization (DTP) and three doses of adult formulation tetanus and dT 2 years earlier. Clinical diagnosis of diphtheria was not made until microbiological examination of specimens confirmed C. diphtheriae biovar gravis (VA01 strain) [Reference Mattos-Guaraldi10]. Multilocus sequence typing (MLST) to study genetic relationships in C. diphtheriae strains isolated in the urban area of Rio de Janeiro showed that the VA01 strain was assigned to an already known sequence type (ST), indicating that it was part of a clonal complex that comprises strains isolated in Canada (ST80) [Reference Viguetti27]. These cases reinforced the potential susceptibility of Brazilian children and adults to diphtheria that may be caused by both endemic and imported clones.
Although in Brazil and Eastern Europe outbreaks mainly occur due to the dissemination of C. diphtheriae biovars mitis and gravis, respectively [Reference Dittman4, Reference Mattos Guaraldi, Hirata and Damasco5], in Maranhão the diphtheria outbreak was caused by C. diphtheriae biovar intermedius. Interestingly, diphtheria cases and deaths also caused by C. diphtheriae biovar intermedius have been documented in previously immunized individuals in India [Reference Phalkey25, Reference Dravid and Joshi28]. By using PFGE it was possible to conclude that five toxigenic strains isolated in the state of Maranhão in 2010 were classified within the same clone or PFGE group. However, MA136 strain, the only one without the tox gene, was classified in a different PFGE type unrelated to toxigenic strains from Maranhão, indicating the circulation of more than one PFGE type of C. diphtheriae biovar intermedius during the outbreak which had taken place in this northern state of Brazil.
The presence of diphtheria bacilli resistant to drugs, frequently used in the treatment of infections from different sources (e.g. penicillin and erythromycin) has been noticed in some countries, including Brazil [Reference Pimenta21]. Penicillin tolerance has been hypothesized to be a cause of treatment failure of C. diphtheriae infections [Reference Von29, Reference Gladin30]. Presently, even considering the breakpoints for S. aureus, we observed a decreased susceptibility to penicillin in C. diphtheriae strains isolated from the patients of municipalities of the state of Maranhão. Microorganisms also showed resistance to tetracycline, as previously observed in Rio de Janeiro [Reference Pimenta21]. C. diphtheriae strains of PFGE types Ia and Ib and II showed 100% susceptibility to erythromycin, linezolid and vancomycin. The representative strain of PFGE type II (MA136 strain) showed susceptibility to most of the antimicrobial agents tested, except clindamycin. Continuous surveys of antibiotic susceptibility of C. diphtheriae, especially in developing countries where diphtheria is endemic and invasive infections may occur remain necessary.
In conclusion, diphtheria remains endemic in Brazil, including the northern state of Maranhão. Unvaccinated or inadequately vaccinated people who travel to countries where diphtheria is endemic may be at increased risk. Our review of diphtheria patients hospitalized in Maranhão suggests that the clinical features of the disease in partially vaccinated patients may still be similar to those that were observed in the pre-vaccine era. However, we cannot exclude the possibility of some cases of diphtheria being misdiagnosed or not notified to the public health authorities due to the lack of knowledge by physicians regarding the clinical and laboratory diagnosis of diphtheria, particularly when patients are not fully protected against diphtheria toxin. The scarcity of epidemiological data concerning diphtheria in developing countries may be due to the fact that doctors and microbiologists are not aware of the possibility of atypical cases of C. diphtheriae infection, including pharyngitis without pseudomembrane [Reference Phalkey25] as well as invasive infections such as pneumonia [Reference Honma31, Reference Trost32], arthritis and endocarditis [Reference Pimenta21, Reference Guran33–Reference Hirata36] and catheter-related infections [Reference Gomes16].
ACKNOWLEDGEMENTS
This is work was supported by FAPEMA, FAPERJ, CNPq, CAPES, SR-2/UERJ, Programa de Núcleo de Excelência (PRONEX) and Programa Nacional de Pós-Doutorado – PNPD (CAPES/MEC).
DECLARATION OF INTEREST
None





