INTRODUCTION
No previous social network studies of injecting drug users (IDUs) or infectious diseases epidemiology have been reported in Australia. In the United States, various authors have employed network methods to produce new insights about HIV transmission among IDUs. Friedman et al. [Reference Friedman1] showed that small sub-networks of HIV-negative IDUs can contain outbreaks in parent networks, Rothenburg et al. [Reference Rothenberg2] explained how specific network structures may facilitate HIV transmission, and Latkin et al. [Reference Latkin3] elucidated relationships between injecting frequency and risk network structure. Kottiri et al. [Reference Kottiri4] found that network characteristics formed part of the explanation for the higher observed HIV prevalences among black IDUs compared to their white counterparts. Most importantly (for the purposes of this paper), Laumann & Youm [Reference Laumann and Youm5] demonstrated that the relatively high prevalence of STI infection in African-Americans compared to other racial groups was explicable by a greater tendency to dissortative mixing (interaction of individuals with widely differing risk behaviour profiles) in their sexual networks.
Between June 2000 and July 2002, the authors conducted a study of the social networks of IDUs which incorporated molecular epidemiology of the hepatitis C virus (HCV). Our study was located in Footscray, a western suburb of Melbourne (the state capital of Victoria, Australia) and home to one of the city's largest illicit drug (principally heroin) markets. Footscray has a diverse ethnic makeup, the largest ethnic group being Vietnamese immigrants who began arriving in Australia in the mid-1970s and their children, many of whom retain a strong ethnic identity despite living in Australia for most or all of their adult lives. Ethnic Vietnamese in Australia are particularly vulnerable to involvement with illicit drugs [Reference Reid6], and are over-represented in opioid treatment [Reference Reid7] and heroin-related arrest statistics [Reference Beyer8]. In Footscray, Vietnamese IDUs comprise a substantial and relatively visible subset of the area's IDUs.
Our aims were to:
• compare patterns of HCV infection in ethnic Vietnamese IDUs and IDUs of other ethnicities (non-Vietnamese);
• compare the social networks of Vietnamese IDUs and non-Vietnamese IDUs in terms of local structure and distribution of risk behaviour for blood-borne virus (BBV) infection;
• test whether the pattern described by Laumann & Youm [Reference Laumann and Youm5] of assortative mixing (interaction between individuals of similar risk profile) with respect to ethnicity, but dissortative mixing with respect to risk behaviour for infection acquisition, holds true for the ethnic Vietnamese minority in our study group.
METHODS
Between September 2001 and July 2002, 199 IDUs completed an extensive questionnaire covering their personal and behavioural characteristics, and were asked to describe members of their injecting network and introduce them to us. Network members were defined as people who had injected drugs with the interviewee, at the same time and in the same location, at least once during the previous 6 months. Pairs of IDUs in which one named the other, and/or the reverse, as an injecting partner are termed dyads; sets of IDUs in which each has reported injecting with at least two others are termed 3-cliques. Dyad and clique membership by Vietnamese and non-Vietnamese IDUs was used to evaluate whether mixing was assortative by ethnicity.
To test whether dissortative mixing with respect to risk behaviour existed in our ethnic Vietnamese relative to non-Vietnamese IDUs, we first assessed risk on the basis of four variables that have been shown to be strongly associated with heightened risk of BBV infection in previous studies and/or are logical risk factors. Our proxies for risk were duration of injecting [Reference Maher9, Reference Weild10], frequency of injection (in the month prior to interview) [Reference MacDonald11, Reference Miller12], frequency of needle-sharing (in the 6 months prior to interview) [Reference Perngmark13, Reference Lamden14], and finally number of injecting partners (in the month prior to interview; analogous to Laumann & Youms' number of sexual partners). We selected Vietnamese IDUs who reported low levels of each behaviour frequency or characteristic (arbitrarily determined by sorting IDUs on each proxy variable and selecting, as near as possible, the low third), then non-Vietnamese IDUs who reported injecting frequency in the same range, calculated the mean for each individual's network members' aggregated risk behaviour, and finally compared the overall means for Vietnamese and non-Vietnamese IDUs.
Venous or fingerprick blood samples were collected from 198 IDUs. All samples were tested for the presence of HCV RNA by the Cobas Amplicor HCV test (Roche, Branchburg, NJ, USA); PCR-positive blood samples were genotyped by a reverse-phase hybridization line probe assay (LiPA, Versant HCV genotype assay; Bayer, Tarrytown, NY, USA) as previously described [Reference McCaw15] Sequencing was carried out on the PCR product using ABI PRISM™ Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA, USA) and the genotype obtained was compared to the LiPA result for each sample. Core sequences (307–328 bp, depending on genotype) were grouped according to genotype and phylogenetic analysis was performed using the PHYLIP package [Reference Feldenstein16]. Genetic distances for each pair of virus samples were calculated using DNAdist; SEQBOOT was used to estimate the statistical significance of the clusters based on sets of genotype-specific sequence data. HCV infections were identified as significantly related if their bootstrap value was ⩾70% [Reference Hershkovitz, Leipe, Baxevanis and Ouellette17].
All data were entered into a relational FileMaker Pro 5 database (Claris, Santa Clara, CA, USA) for ongoing management. Network measurements were made using UCINET [Reference Borgatti18] and exported to SPSS, in which all statistical analyses were carried out. For more details of our research methods, see Aitken et al. [Reference Aitken19].
RESULTS
Of 199 interviewed IDUs, 49 were Vietnamese and 150 non-Vietnamese. In univariate comparisons of means, Vietnamese IDUs were significantly younger than non-Vietnamese IDUs (26·7 vs. 31·0 years; 95% CI for difference −6·3 to −2·3). A higher proportion of non-Vietnamese than Vietnamese participants were living in transient accommodation or other uncertain circumstances, or on the street (41·3% vs. 26·5% – a difference of only weak statistical significance; P=0·06).
Network measures
In total, 197 of our participants were members of one large connected component containing 577 dyadic relationships. The remaining two IDUs (both of Australian ethnicity) were isolates – they did not nominate any other interviewed IDUs as network members (injecting partners), and were nominated by none. Overall, 70·4% of people nominated as injecting partners by study participants were ultimately interviewed.
The 49 ethnic Vietnamese IDUs nominated more network members than did the 150 non-Vietnamese (5·4 vs. 4·3, 95% CI for difference 0·2–1·8), and more of their members were interviewed (4·2 vs. 2·9, 95% CI for difference 0·5–2·0). As a result, mean degree (the average number of injecting links per IDU) is significantly greater for Vietnamese than non-Vietnamese participants (8·0 vs. 5·6, 95% CI for difference 0·9–4·0), denoting a higher level of connectedness than among the non-Vietnamese.
Given the higher mean degree for Vietnamese participants just described, we expected to find a relatively high number of dense sub-networks involving IDUs of Vietnamese ethnicity. Of 109 3-cliques discernible in the network, 26 (23·9%) are Vietnamese-only, 53 (48·6%) non-Vietnamese-only, and 30 (27·5%) mixed; thus Vietnamese IDUs are involved in over 50% of all 3-cliques in the network, a significantly higher percentage than expected on the basis of their proportion in the study group (24·6%; OR 3·2, 95% CI 1·9–5·5).
Prevalences of HCV and exposure
Forty-four of 49 (89·8%) ethnic Vietnamese IDUs were anti-HCV-positive, vs. 128/149 non-Vietnamese (86·5%); 39/48 (81·3%) Vietnamese and 100/141 non-Vietnamese (70·9%) had detectable HCV RNA. Eight samples from non-Vietnamese IDUs and one from a Vietnamese IDU could not be tested for HCV RNA. Neither comparison of prevalence across Vietnamese and non-Vietnamese IDUs produced a significant difference (OR 1·44, 95% CI 0·5–5·2; OR 1·8, 95% CI 0·8–4·5 respectively).
Molecular epidemiology
Phylogenetic analysis identified three clusters (two pairs and a septuplet) of related infections that included Vietnamese and non-Vietnamese IDUs; they can be broken down into (2+7!/5!2!=) 23 discrete IDU pairs (combinations of two), involving five Vietnamese and six non-Vietnamese IDUs. Fourteen of those 23 pairs with related HCV infections contain a Vietnamese and a non-Vietnamese IDU. Although it is obvious that injecting relationships across the ethnic divide must have existed for these related infections to occur, none of the 14 injecting relationships involving IDUs of discordant ethnicities (or indeed, the remaining nine involving IDUs of matching ethnicities) implied by the molecular data was validated by our social network data. In particular, none of the five Vietnamese IDUs nominated any of the six non-Vietnamese IDUs with related HCV infections as injecting partners, nor did the reverse occur. Table 1 provides comparative data on dyadic relationships and related HCV infection pairings by ethnicity.
Table 1. Dyadic relationships and related HCV infection pairings by ethnicity
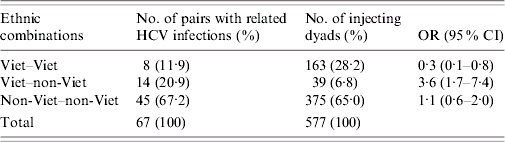
Viet, Vietnamese; OR, Odds ratio; CI, confidence interval.
Table 1 shows that Vietnamese–non-Vietnamese IDUs account for a significantly higher proportion of related HCV infections (detected via molecular epidemiology) than of dyads (mapped from IDUs' self-reported injecting relationships). Conversely, many fewer Vietnamese–Vietnamese related infections were detected than expected from the number of Vietnamese–Vietnamese social dyads mapped. Note that 93·2% of injecting dyads, and 79·1% of pairs of related infections, consist of IDUs of concordant racial/ethnic group, thus – along with the clear ethnic divisions in clique membership reported earlier – confirming that mixing is highly assortative with respect to ethnicity in this group of injectors.
Risk behaviour
On average, Vietnamese IDUs had been injecting drugs for fewer years than non-Vietnamese IDUs (6·4 vs. 11·5 years, 95% CI for difference −7·0 to −3·2); however, Vietnamese IDUs were injecting much more frequently on average than their non-Vietnamese counterparts in the month prior to interview (49·2 vs. 35·6 times, 95% CI for difference 0·9–26·4). Vietnamese IDUs were injecting with a significantly larger mean number of HCV PCR-positive (i.e. potentially infectious) network members than non-Vietnamese IDUs (2·9 vs. 1·9, 95% CI for difference 0·4–1·6).
Duration of injecting, frequency of injecting, frequency of needle-sharing, and number of injecting partners were used to test whether mixing with respect to risk behaviour was dissortative (low-risk mixing with high-risk) in our Vietnamese IDUs relative to non-Vietnamese participants. Table 2 contains comparison data.
Table 2. Comparisons of the risk behaviours of network members of low-risk Vietnamese and low-risk non-Vietnamese participants
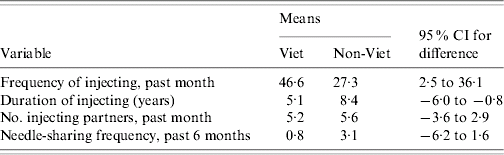
Viet, Vietnamese; CI, Confidence interval.
Table 2 shows that the mean reported injecting frequency of the network members of Vietnamese IDUs who themselves reported low injecting frequency (<12 times in the month prior to interview, compared to the median of 30) was significantly greater than that reported by the network members of non-Vietnamese IDUs reporting injecting frequency in the same range. That pattern was reversed with respect to duration of injecting – the durations reported by the network members of Vietnamese IDUs who had been injecting for ⩽3 years (compared to a median of 8·1 years) were significantly shorter on average than those reported by network members of non-Vietnamese IDUs. No significant difference existed between the ethnic groups in the number of injecting partners reported in the past month by low-risk IDUs (‘low’ meaning two or fewer partners in the months prior to interview, compared to the overall median of five). No difference was detected in mean reported frequencies of needle-sharing for the network members of low-risk individuals (not surprising as 63% of interviewees reported no needle-sharing in the 6 months prior to interview).
DISCUSSION
A relatively high level of interconnectedness is not an unusual finding for a geographically concentrated group of recent immigrants and their offspring [Reference Hauff and Vaglum20–Reference Viviani22]. Our Vietnamese IDUs were part of a large, established ethnic minority community, and we employed two Vietnamese-speaking researchers – one of whom was also ethnically Vietnamese – to recruit and interview them. Thus, the significantly higher mean degree (number of injecting links) per Vietnamese IDU may be partly due to our focus on this IDU subgroup and their geographical ‘loyalty’ relative to non-Vietnamese IDUs, who were more likely to be living in transient accommodation. Opposing that interpretation is the fact that the proportions of network members nominated by Vietnamese and non-Vietnamese IDUs who were actually interviewed were not significantly different, suggesting that the higher numbers of network members of Vietnamese IDUs both nominated and interviewed were not artificially influenced by our research methods. The logical corollary of higher mean degree in a network of IDUs is greater opportunity for infectious disease transmission; the lack of a statistically significant difference between HCV prevalences for Vietnamese and non-Vietnamese IDUs in this study may be a result of the high overall prevalence of HCV exposure among Australian IDUs, meaning very large participant numbers are required to demonstrate significant differences between ethnic groups. HIV prevalence was not measured in our study, but a study of a group of Vietnamese IDUs that overlapped ours [Reference Nguyen23] produced an HIV prevalence of 2·4%, much higher than the rates of 0·9–1·4% typically found in Australian IDUs of unspecified or mixed ethnicities [Reference Thein24]. Vietnamese IDUs are overrepresented in Victorian HIV notification data; of IDUs diagnosed with HIV between January 1999 and March 2003, 41% (14/34) were born in Vietnam, while the proportion of Vietnamese-born in the Victorian community was 1·5% [Reference Hocking25, Reference Guy26]. It is possible, when combined with denser social network ties, the higher rates of injecting frequency reported by our Vietnamese IDUs (and especially by low-risk Vietnamese IDUs), while insufficient to significantly inflate already very high HCV prevalences, are nonetheless responsible for their high HIV prevalence relative to non-Vietnamese IDUs.
With respect to mixing and ethnicity, our data mirror the findings of Kottiri et al. [Reference Kottiri4] and are in line with the assortative mixing described by Laumann & Youm [Reference Laumann and Youm5] in that our participants overwhelmingly chose injecting partners of the same broad racial/ethnic category. Over 90% of injecting dyads were comprised of Vietnamese–Vietnamese or non-Vietnamese–non-Vietnamese IDUs, as were nearly 80% of IDU pairs with related infections; membership of 3-cliques (dense network sub-regions) were similarly strongly divided along ethnic lines. Our network data suggest – although undermined somewhat by the phylogenetic data – that avenues for transmission of HCV infections across this ethnic division are rare. No occurrences of genotypes 6a or 7a (highly characteristic South-East Asian genotypes) in non-Vietnamese participants, which would have signalled transmission across the ethnic divide, were observed. Highly assortative mixing by ethnicity means that BBV infections are likely to remain within each ethnic group.
There are two possible explanations for the mismatch between the proportions of Vietnamese–non-Vietnamese injecting dyads and related infections. The first is that third parties not included in our research may have provided a pathway for the virus rather than there being any direct transmission between our participants. Slightly fewer than 30% of the network members nominated by our participants were not interviewed, so it is likely that at least 150 dyadic injecting relationships remained hidden from us. An alternative explanation is that these HCV transmissions could have occurred when Vietnamese and non-Vietnamese IDUs injected together as part of a heroin transaction. (It is common for an IDU seeking a large amount of heroin to be introduced by a street dealer to a higher-level supplier in return for a small share, which is injected communally immediately after purchase. Four of the five Vietnamese IDUs known to have injected with non-Vietnamese IDUs were engaged in selling heroin when recruited.) Such interactions are usually brief, involving little social interaction above that required to complete the transaction, and infrequently repeated; thus the likelihood of seller or buyer including the other as an injecting network member is small. This hypothesis is supported by the relatively small proportion of social dyads that included a Vietnamese and non-Vietnamese IDU. The mismatch between related infections and dyads with discordant ethnicities seems to confirm that much more social (injecting) interaction was taking place between Vietnamese and non-Vietnamese IDUs than we were able to capture.
It should be remembered that our data are incomplete on three fronts. Not all of the IDUs nominated as injecting partners were interviewed (77·8% and 67·4% of nominees of Vietnamese and non-Vietnamese participants respectively), and there were undoubtedly cases in which interviewed IDUs were nominated as injecting partners but not sufficiently well identified to enable dyads to be confirmed. In addition, phylogenetic analysis can only determine the statistical likelihood of relatedness of two HCVs, not identify the source of transmission; thus, while two participating IDUs can be deemed to carry a related HCV infection, one or both may have been infected by a non-interviewed third party, or by a participant with whom an injecting relationship existed but could not be recorded for study purposes. A further limitation of our study is that, being a network study, no attempt was made at sample representativeness (always highly dubious in IDU studies in any case), so our results may not be generalizable to other situations and populations.
Adapting Laumann & Youm's methods produces a somewhat confused picture of mixing with respect to risk for our study group. Low-risk Vietnamese IDUs appeared to choose relatively high-risk partners in terms of injecting frequency compared to low-risk non-Vietnamese IDUs, but chose relatively low-risk partners when duration of injecting was considered. From these results, we are unable to say that dissortative mixing occurs with respect to BBV risk behaviours among ethnic Vietnamese IDUs relative to the non-Vietnamese IDUs in our study group. Nevertheless, higher rates of injecting across all our Vietnamese participants compared to non-Vietnamese and higher rates of injecting with HCV carriers, plus indications of higher HIV prevalence in ethnic Vietnamese IDUs in general, suggest that Vietnamese IDUs are more vulnerable to BBV infection.
ACKNOWLEDGEMENTS
The authors are grateful to Ms Rhonda McCaw (Victorian Infectious Diseases Reference Laboratory, North Melbourne, Australia) for conducting the phylogenetic analysis, and to Jenny Kelsall, Michael Kerger and Hoang Nguyen for collecting the data. The project on which this article is based was funded by the National Health and Medical Research Council of Australia (project grant no. 111701).
DECLARATION OF INTEREST
None.




