The haemoprotozoan parasite, Trypanosoma evansi, is the causal agent of a disease in domestic animals, which is popularly known as ‘surra’. However, the disease is not only restricted to animals. Recently, the infection has also been reported in humans in India [Reference Joshi1], which established the zoonotic importance of the disease. Veterinarians and clinicians are responsible for the handling of clinical cases which are suspected of being surra infections. Hence, there is a need to know the prevalence of the disease in suspected animals and also within the different regions of India in order to define appropriate control programmes against the disease. However, the diagnosis of T. evansi infection is problematic, mainly because of its varied and non-specific clinical manifestations in enzootic areas. Parasite detection techniques by examination of Giemsa-stained blood smears (GSBS) are not always reliable for current infections when the level of parasitaemia remains low and fluctuates, particularly during the chronic stage of infection. Hence, in the present study, as an alternative method of diagnosis of suspected cases of surra, the mouse inoculation test (MIT) and enzyme-linked immunosorbent assay (ELISA) were used in addition to examination of GSBS, for the detection of surra in clinically ill and suspected cases of cattle, buffaloes and horses.
This study was undertaken on samples from buffaloes, cattle and horses which were proven to be clinically ill and suspected of T. evansi infection as observed by clinical signs. Blood samples from these clinically ill animals, suspected of surra, were collected aseptically and each sample was processed for the preparation of blood smears for Giemsa stain, MIT and for collection of serum. For the detection of anti-T. evansi antibodies in suspected buffaloes, cattle and horses, an indirect ELISA was used as described previously [Reference Luckins2, Reference Jithendran, Rao and Mishra3]. For the detection of antibodies against T. evansi in serum samples collected from suspected buffaloes and cattle, crude whole cell lysate (WCL) antigen prepared from trypanosomes isolated from buffalo origin were used. Similarly crude WCL antigen prepared from trypanosomes isolated from horse origin was used for the detection of T. evansi in serum samples from suspected horses. Sera of cattle, buffaloes and horses, found positive for T. evansi infection either in blood-smear examination or in MIT, or in both, were used as positive sera. Sera collected from non-enzootic areas that was shown to be negative for T. evansi infection by both GSBS and MIT, and which was also negative by an indirect ELISA during a repeated chequerboard titration, were used as negative sera. For each ELISA test, crude sonicated buffalo antigen, 1·35 μg protein/well and horse antigen 1·26 μg protein/well, were coated. The obtained optical density (OD) values were expressed as percent positivity (PP) by dividing the average OD value of the sample by the median value of the OD values of the strong positive control, and multiplying by 100. The ELISA cut-off value was determined as the mean PP obtained from the negative sera multiplied by 2.
A total of 102 clinically suspected animals (38 buffaloes, 32 cattle, 32 horses) were examined by GSBS, MIT and the antibody detection ELISA (Ab-ELISA) to determine the prevalence of the disease. Twenty-two cattle from an organized cattle farm in a non-endemic area, which were clinically healthy, were screened by both the parasitological and the serological test for comparison. The examination by GSBS detected 5·3%, 9·4% and 40·6% infections in buffaloes, cattle and horses, respectively. Low levels of parasitaemia were observed in cattle and buffaloes and high levels were observed in horses. The MIT detected 18·4%, 15·6% and 46·9% infections in buffaloes, cattle and horses, respectively. The Ab-ELISA detected anti-trypanosomal antibody in 42·1%, 43·8% and 65·6% clinically ill buffaloes, cattle and horses, respectively (Table 1). All healthy animals were found negative by parasitological examination and Ab-ELISA test.
In the present study cattle, buffaloes and horses incubating T. evansi might have enabled the production of antibodies by allowing sufficient time after infection. In contrast, due to the fluctuating level of parasitaemia, T. evansi might not be detected during examination by GSBS and MIT. This might be the reason for the lower prevalence observed by examination of GSBS and MIT, in comparison to the Ab-ELISA test. A comparison of the prevalence of this infection with the Southern part of India shows a low prevalence of T. evansi in cattle (1·4%) and buffaloes (2·7%) as recorded by GSBS [Reference Das, Nandy and Mohankumar4].
Table 1. Detection of Trypanosoma evansi infection in clinically ill and suspected cases of buffaloes, cattle and horses by examination of GSBS, MIT and indirect ELISA
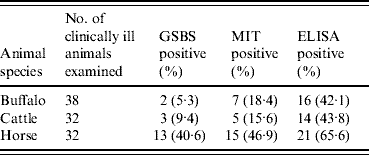
GSBS, Giemsa-stained blood smear; MIT, mice inoculation test; ELISA, enzyme-linked immunosorbent assay.
Compared to Eastern India, the disease is quite prevalent in Northern India. Eight outbreaks were recorded in cattle and buffaloes during a 5-year period and it has been reported that 295 animals were affected of which 142 died [Reference Batra, Kumar and Kulshreshta5]. Circulating antigen of T. evansi was detected by antigen detection ELISA (Ag-ELISA) in 55·7% infected/suspected buffaloes against 19·2% positivity by wet blood film (WBF) examination. In the case of horses 56·3% infection was detected by Ag-ELISA and 23·8% by WBF. Ab-ELISA detected 35·9% cases in buffaloes and 37·5% in horses from Northern India [Reference Singh6]. Thus, it has been shown that the prevalence of T. evansi infection is greater in Northern India than in the Eastern Region of India. In a recent study, in randomly collected sera samples of cattle, buffaloes and horses, an antibody detection test showed the presence of anti-T. evansi antibodies in 20·7% and 11·1% sera samples of slaughtered cattle and buffaloes, respectively, in 5·7% of sera samples of cattle collected from villages and in 10% of sera samples of horses [Reference Laha and Sasmal7]. Recently, it has been reported that T. evansi can be established in an unnatural host, the pigeon [Reference Mandal, Laha and Sasmal8]. It has also been established that a patient was infected with T. evansi, due to a host genetic defect, i.e. the lack of apolipoprotein L-I [Reference Vanhollebeke9]. Moreover, another species of Trypanosoma, i.e. Trypanosoma lewisi, which is generally non-infective for humans, has recently been reported to infect human patients in India [Reference Kaur10]. From the present study 50% of clinically ill and suspected animals were positive for T. evansi infection. Due to the fact that T. evansi has zoonotic significance, veterinarians and clinicians handling the clinical material suspected of surra infection should do so with care.
In conclusion, a considerable number of animals in the Eastern Region of India, which were suspected of surra infection, are infected with T. evansi and awareness of this should be raised especially in high-risk workers including veterinarians and farmers.
ACKNOWLEDGEMENTS
The authors are grateful to the Vice Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata – 700037, West Bengal India, for providing the facilities to undertake this investigation.
DECLARATION OF INTEREST
None.



