INTRODUCTION
Despite the marked decrease in HIV-related mortality and morbidity since the introduction of highly active antiretroviral therapy (HAART), the best therapeutic strategy has yet to be established. In patients with CD4 cell counts >350 cells/mm3 and HIV viral loads <100 000 copies/ml, current guidelines generally recommend deferring therapy, given the low absolute risk of AIDS-defining clinical events before this threshold [Reference Hogg1–Reference Hulgan6] and the risk of both HIV resistance and antiretroviral-related toxicity.
On the other hand, reaching and maintaining a CD4 level >500/mm3 for at least 5 years on HAART reduces mortality to a level similar to that in those without HIV infection [Reference Lewden7]. Since the likelihood of reaching a CD4 level >500/mm3 is higher in patients starting HAART at high CD4 levels [Reference Garcia8–Reference Moore and Keruly10], it can thus be speculated that early HAART could be associated with a clinical benefit. The improvements in tolerance to and the efficacy of recent antiretroviral regimens, associated with a potential clinical interest of better virological control [Reference Emery11], and a lower risk of HIV resistance, could strengthen this approach [Reference Hulgan6, Reference Chaisson, Keruly and Moore12, Reference Phillips13].
Besides the ‘when to start question’, the ‘what to start with’ question needs to be answered. Most therapeutic trials observed that the efficacy of first-line HAART including two nucleoside analogue reverse transcriptase inhibitors (NRTI) plus either a boosted protease inhibitor (PI-based HAART) was similar to that using a non-nucleoside reverse transcriptase inhibitor (NNRTI-based HAART). However, the different safety profiles [Reference Friis-Moller14, Reference Madden15], the potential difference in immunological outcome [Reference Bartlett16] and the difference regarding the genetic barrier may induce significant differences over time.
Two recent large cohort studies concluded that, in the case of immediate HAART, not only were patients with CD4 levels between 350 and 450–500/mm3 at a lower relative risk of AIDS and/or death [17, Reference Kitahata18], but also that those with CD4 >500/mm3 were at a lower relative risk of death [Reference Kitahata18]. However, no randomized trial tested specifically the ‘when and what to start’ question. In addition, HIV infection has become a chronic disease. Thus, we need to identify the best strategy while bearing in mind the positive and negative consequences over a period of at least 10 years. Decision analysis using Markov modelling is known to be the most appropriate method in such cases. This is why it was applied rather frequently in the field, mainly to perform cost-effectiveness analyses [Reference Tebas19, Reference Vijayaraghavan20]. However, is also implies that enough data on the main inputs are available. In a previous study, we applied a Markov model to a prospective cohort of HIV-infected patients on different HAART strategies to assess the factors for clinical and immunological evolution [Reference Binquet21]. In the present study, our aim was to build a decision tree including the different antiretroviral strategies that could be adopted, and then by using the transitional probabilities drawn from our Markov model, to determine the best strategy in HIV-infected patients according to their baseline characteristics.
METHODS
Study design
This study was conducted using a decision analysis design [Reference Weinstein and Fineberg22]. We used a decision tree to simulate the different treatment strategies and final outcomes for virtual patients according to their baseline characteristics and intermediate evolution (Figs 1, 2).
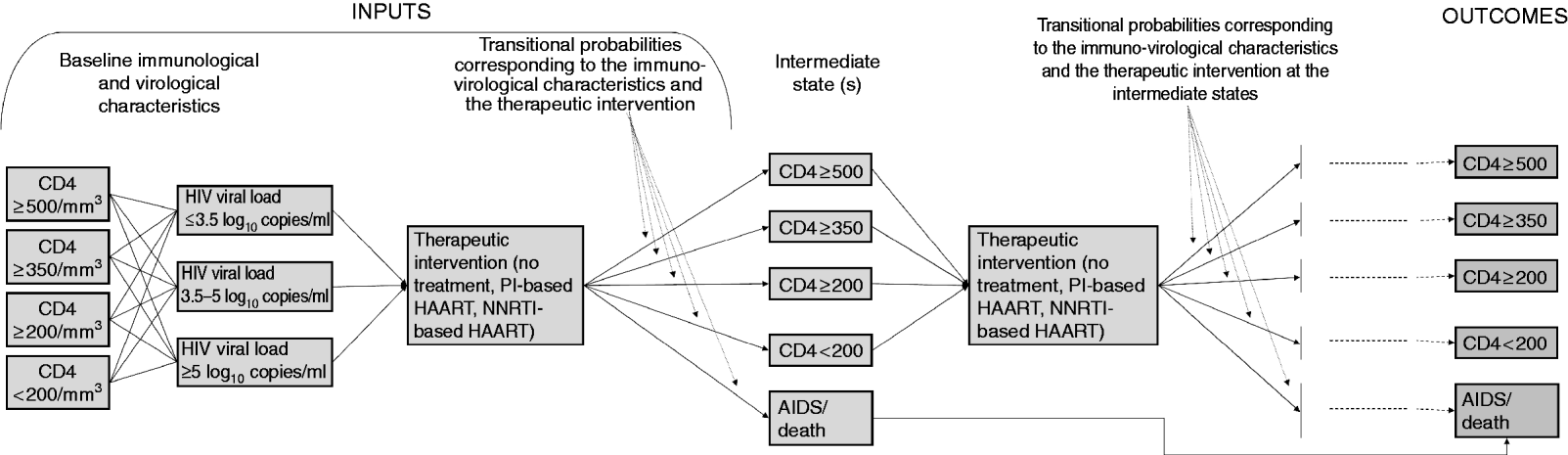
Fig. 1. Flow diagram synthetically representing the inputs, the general methodology and the outcomes (See also Fig. 2). HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
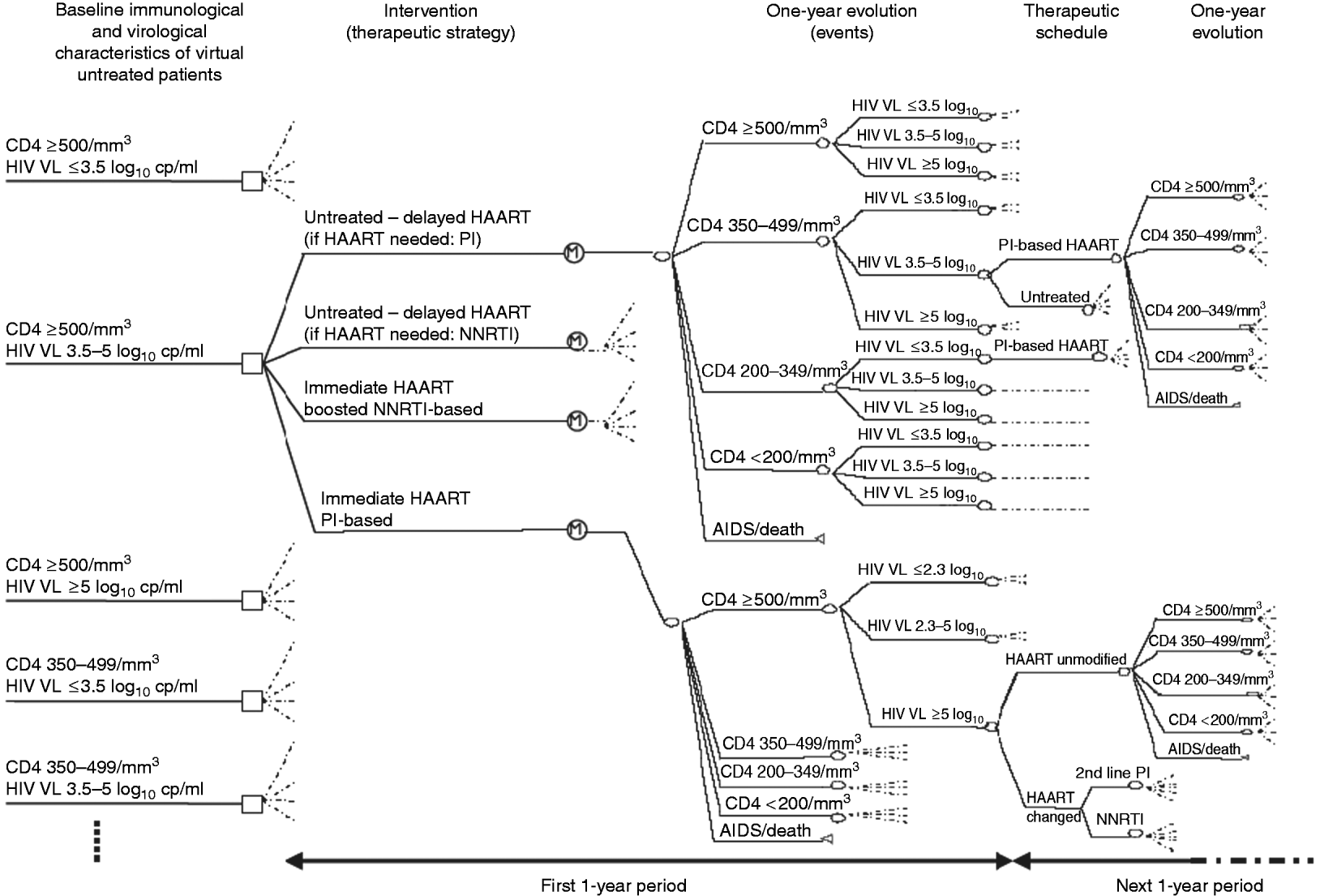
Fig. 2. Schematic representation of a part of the decision tree. From the immunological and virological baseline characteristics of the patients (left part), four different antiretroviral strategies can be used (e.g. as shown with patients with baseline CD4 >500/mm3 and HIV viral load between 3·5 and 5 log10 copies/ml, middle part). According to the strategy used and the characteristics of the patients, subsequent clinical, immunological and virological events occur with different probabilities (estimated in a previous observational study). As examples, subsequent potential evolutions and therapeutic schedules are shown in the right upper part for initially untreated patients, and in the right lower part for those immediately treated with boosted PI-based HAART. cp/ml, copies/ml; HIV VL, HIV viral load; HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The decision tree included the alternative therapeutic strategies of interest. Then, the potential subsequent events of interest (clinical, immunological, therapeutic) were added. The evolution of the virtual patients towards the different events was simulated over repeated 1-year periods. For each of the subsequent events, 1-year probabilities of event occurrence were applied according to the characteristics of the virtual patients at the beginning of each period. These probabilities were obtained from a previous study [Reference Binquet21]. Utility values were then associated with the different events (the greater the interest of an event, the higher the utility value). The combination of utility values with the distribution of probabilities allowed us to calculate an intermediate expected value (or score). The process was repeated ten times using a Markov process to simulate evolution over 10 years and to calculate a final score for each strategy (the higher the final score, the better the strategy). All analyses were performed with decision analysis software (TreeAge ProTM 2006 HealthCare, TreeAge Software Inc., USA).
Strategies compared and decision tree structure
Four main strategies were compared: (1) no initial treatment, potentially followed by NNRTI-based HAART when the CD4 count fell to <350 cells/mm3; (2) no initial treatment, potentially followed by boosted PI-based HAART when the CD4 count fell to <350 cells/mm3; (3) immediate NNRTI-based HAART; and (4) immediate boosted PI-based HAART. It was assumed that HAART once started would never be stopped, and that the initial HAART schedule would have to be changed in some patients, in particular for tolerance or efficacy issues. In the latter, it was also assumed that a patient would receive only one NNRTI-based HAART during follow-up. For all HAART schedules, boosted-PI or NNRTI were associated with a backbone of two NRTIs.
Clinical and immunological events
The events in the decision tree were (1) to have a CD4 count ⩾500/mm3, or (2) to have a CD4 count ⩾350 and <500/mm3 (350–499), or (3) to have a CD4 count ⩾200 and <350/mm3 (200–349), or (4) to have a CD4 count <200/mm3, without experiencing clinical progression (AIDS or death); or (5) to experience clinical progression to AIDS or death (whatever the CD4 count).
Probabilities of evolution
The 1-year transitional probabilities for each of the potential events were estimated in a previous study [Reference Binquet21] from the ICONE cohort using a time-homogeneous Markov model [Reference Alioum and Commenges23]. Briefly, this cohort included 2126 consecutive HIV-1 infected adults (>15 years) seen for the first time and prospectively followed in one of the six participating centres from July 1996 to June 2004. The baseline characteristics of these patients are presented in Table 1. One-year transitional probabilities from one immunological state to another were estimated from the 44 021 transitions observed in this cohort (Table 2) according to the HIV viral load, the HAART status, the type of HAART, and the number of previous antiretroviral treatments. In HAART-treated patients, this cohort also allowed us to estimate the likelihood that a patient would remain on the same therapeutic schedule during the 1-year period, or change to another one (from one boosted-PI to another boosted-PI, from NNRTI-based to boosted-PI, or from boosted-PI to NNRTI-based for NNRTI-naive patients).
Table 1. Baseline characteristics of the 2126 patients included in the study (ICONE group, 1996–2004)
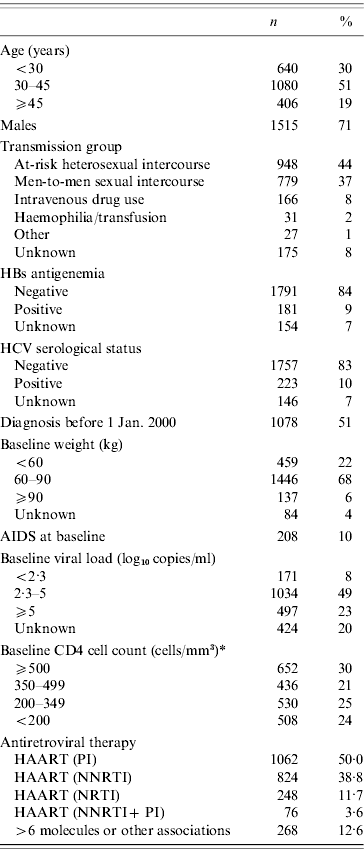
HAART, Highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
* Missing values of CD4 cell count were imputed using previous or next measurement, if the time-lapse between the two follow-ups was <3 months for patients without any antiretroviral treatment, or <1 month for treated patients.
Table 2. Number of transitions from one stage to another (ICONE group, n=2126, 1996–2004)

Utilities
A utility value was associated with each event as a measure of relative preference, ranging from 0 (highly unwanted) to 1 (highly desired). In the main analysis, a utility value of 1 was associated with a CD4 count ⩾500/mm3 (i.e. in this analysis the only therapeutic goal) and a value of 0 was associated with all the other events. A secondary analysis was performed attributing a utility value of 0 to AIDS or death (in this case the only event to avoid) and 1 for all the states without clinical progression to AIDS or death.
In another secondary analysis, we used life expectancy as a measure of each outcome's ‘utility’. The DEALE method was used [Reference Beck, Kassirer and Pauker24]. Since our target population comprised subjects aged 35 years infected with HIV, we used the data from the ICONE cohort to estimate the specific mortality rates for each CD4 state. The general mortality rate (1/life expectancy) was derived from the data of the Institut National de la Statistique et des Etudes Economiques (INSEE). The specific and the general mortality rates were added to obtain the overall mortality rate, and thus overall life expectancy (1/overall mortality rate). By doing this, life expectancies were 23, 35, 38 and 39 years, for 35-year-old patients with a baseline CD4 count of <200/mm3, 200–349/mm3, 350–499/mm3, and ⩾500/mm3, respectively. A penalty was then applied for each year spent in a state <500/mm3 (penalties of 0·25, 0·5, 0·75 for the immunological states 350–499, 200–349, <200 CD4/mm3, respectively) to indirectly take into account the quality of life.
Decision analysis
In order to identify the most efficient strategy, the final expected value (or score) for each strategy was calculated by cumulating the intermediate 1-year period scores, weighted by the probability of beginning the 1-year period with these characteristics. The best strategy corresponded to the one with the best score at 10 years or the best expected life expectancy.
Sensitivity analyses
To assess the stability of the results, sensitivity analyses were performed by speculating that HAART efficacy may have improved in the last years. The 1-year transitional probabilities of reaching a higher immunological state (or staying at the same immunological level for the CD4 ⩾500/mm3 state) were thus increased by 1–8%, and in parallel the transitional probabilities of reaching a lower immunological state were decreased by 1–8%.
RESULTS
Expected outcomes according to the different antiretroviral strategies
The expected results of the different antiretroviral strategies according to baseline CD4 count and HIV viral loads are shown in Table 3. In delayed initiation of HAART, the median delays before starting HAART, estimated from the decision tree, are summarized in Table 4.
Table 3. Decision tree analyses results of the different antiretroviral strategies: 10-year expected values (scores) according to baseline CD4 counts and HIV viral loads
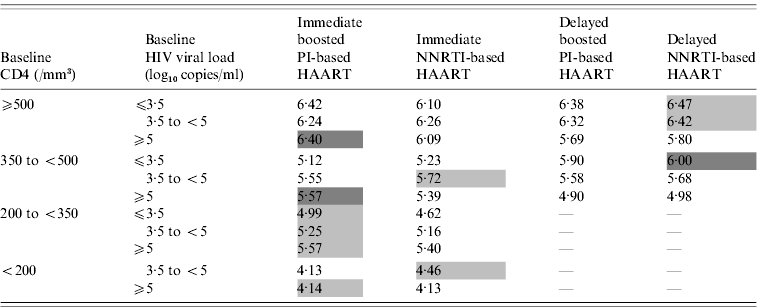
HAART, Highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, Protease inhibitor.
The higher the score, the better the outcome. Best antiretroviral strategies for each baseline CD4 and HIV viral load stratum are indicated in light grey (difference ⩽10% with the worst strategy) or dark grey (difference >10% with the worst strategy).
Table 4. Median time to HAART initiation according to baseline characteristics in case of delayed HAART
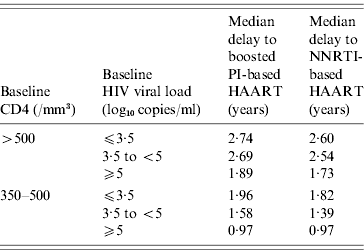
HAART, Highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, Protease inhibitor.
The higher the baseline CD4 state, the higher the expected value, whatever the antiretroviral strategy used. For baseline CD4 ⩾500 and 350–499/mm3, immediate HAART was associated with a better outcome in patients with a baseline HIV viral load ⩾5 log10 copies/ml, the score for delayed strategies being 10% lower than that for the best strategy (immediate boosted PI-based HAART). In contrast, no clear difference was observed in patients with baseline CD4 ⩾350/mm3 and baseline HIV viral load <5 log10 copies/ml. Delayed HAART was even found to perform better than immediate HAART in patients with baseline CD4 350–499/mm3 and HIV viral load ⩽3·5 log10 copies/ml. When HAART was implemented immediately, baseline HIV viral load did not influence the outcome, whereas with delayed therapy, the higher the baseline HIV viral load, the worse the outcome.
Starting immediate antiretroviral treatment with boosted PI-based rather than NNRTI-based HAART was associated with a slightly better outcome in patients with baseline HIV viral load ⩾5 log10 copies/ml, whatever their baseline CD4 count (Table 3).
The direction and the magnitude of these differences were similar when attributing a utility of 0 for AIDS or death and 1 for all other situations.
Life expectancy
A difference of >1 year (for a 35-year-old patient) was observed in favour of immediate PI-based HAART in patients with CD4 ⩾350/mm3 HIV viral load ⩾5 log10 copies/ml (33·2 vs. 30·1 years in case of delayed HAART). Conversely, life expectancy was 1 year longer with delayed HAART than with immediate HAART for patients with baseline CD4 of 350–499/mm3 and HIV viral load ⩽3·5 log10 copies/ml (34 vs. 33 years).
In case of immediate HAART, although no clear difference in life expectancy was observed for patients with baseline immunological states ⩾500/mm3 (mean 33·1 years) and 350–499/mm3 (33 years), they were higher than expected in those with baseline immunological states of 200–349/mm3 (32·6 years, mean difference 0·5 year) and <200/mm3 (29·8 years, mean difference 3·3 years).
Sensitivity analysis
Increasing the transitional probability of passing from one immunological state to a better one by 1–8% percent and decreasing the transitional probability of passing from one immunological state to a worse one also by 1–8% did not modify the ranking of the different strategies for patients with baseline CD4 ⩾350/mm3 and HIV viral load ⩽3·5 log10 copies/ml. In contrast, in patients with baseline CD4 ⩾350/mm3 and an HIV viral load between 3·5 and 5 log10 copies/ml, a relative increase of 4·3% in the transitional probability did modify the ranking of the different strategies, favouring immediate vs. delayed HAART (Fig. 3).
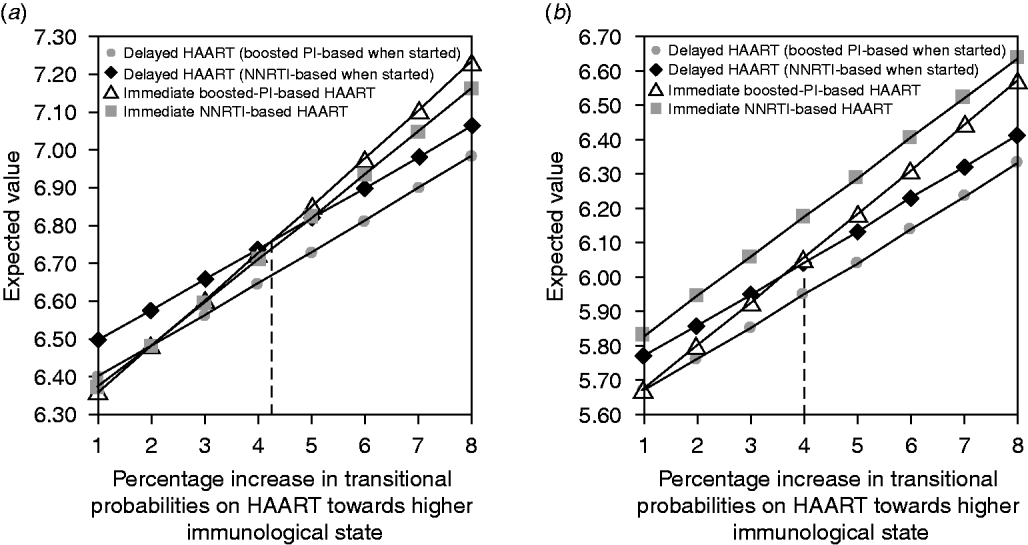
Fig. 3. Sensitivity analysis by increasing the transitional probabilities from one immunological stage to a better one (and symmetrically by decreasing the transitional probabilities to a worse one) from 1% to 8% in patients with baseline HIV viral load between 3·5 and 5 log10 copies/ml. (a) Baseline CD4 >500/mm3 or (b) between 350 and 500/mm3, according to the antiviral strategy used.
DISCUSSION
In the ‘when to start’ and ‘what to start with’ debate, decision tree analysis, which has already been used in the field of HIV infection [Reference Tebas19], may be a useful tool, Indeed, it will allow clinicians to model therapeutic approaches and subsequent outcomes in a real life setting, and may help to build a hierarchy of strategies in order to determine the best one to use in clinical practice [Reference Weinstein and Fineberg22]. It uses the transitional probabilities of evolution to a different state according to different covariates, which could not be obtained directly from classical time-to-event models, such as the Cox proportional hazard model. In our study, these transitional probabilities were drawn from a real-life prospective cohort, the characteristics of which are consistent with other main HIV cohorts [Reference Binquet21]. For example, time to HAART initiation in the case of a delayed strategy in our decision tree analysis (e.g. from 1·73 to 2·74 years for patients with baseline CD4 ⩾500/mm3) is consistent with the median 60–80/mm3 per year CD4 decline slope usually reported [Reference Piroth25–Reference Egger27]. Moreover, it is of interest that changing the values of the different possible events did not significantly modify the results obtained, underlining the high stability of the decision analysis results.
The first result is that immediate HAART for patients with a baseline HIV viral load >5 log10 copies/ml was associated with a better immunological and clinical outcome than in those with delayed HAART. In contrast, delaying HAART appears to be the best option in patients with a baseline CD4 count >350/mm3 and a low baseline HIV viral load (⩽3·5 log10 copies/ml), since the extreme hypothesis of an additional relative increase of up to 8% in the likelihood of clinical and immunological improvement on HAART did not change the ranking of the different strategies.
In patients with CD4 ⩾350/mm3 and an HIV viral load between 3·5 and 5 log10 copies/ml, no clear difference was observed. The long-term potential toxicity of HAART [Reference Friis-Moller14, Reference Madden15] could thus lead to a preference for delayed HAART in these patients, even though it has recently been shown that the higher the CD4 count at HAART initiation, the lower the risk of HAART-related side-effects such as renal insufficiency, peripheral neuropathy and anaemia [Reference Lichtenstein28]. Moreover, a relative increase of at least 4·3% in response to HAART made immediate HAART preferable to delayed strategies. This increase probably reflects the progress made during recent years in the management and increasing virological efficacy of HAART [Reference Kitahata18, Reference Ewings29, Reference Lima30].
Second, no clear difference in clinical and immunological outcome was found between boosted-PI and NNRTI-based HAART, even though there was a trend towards a benefit of boosted-PI HAART in patients with a high baseline HIV viral load (⩾5 log10 copies/ml). Rather than benefiting from a mild ‘direct’ immunological gain [Reference Bartlett16], patients with high levels of HIV replication first treated with low genetic barrier drugs, such as NNRTIs, could be at a disadvantage because of the relative weight of lack of adherence and of subsequent selection of HIV mutations [Reference Lima30]. On the other hand, it could be advocated that NNRTIs may be associated with faster viral decay and greater efficacy at high viral loads [Reference Riddler31], and thus contribute to the lack of difference in patients on delayed HAART.
Of interest, the higher the baseline CD4 count, the better the expected outcome, whatever the strategy used. The baseline CD4 count still appears to have an impact on subsequent immunological and clinical evolution in patients in the HAART era [Reference Abrahamowicz and Mackenzie32], probably because the likelihood of reaching a CD4 count >500/mm3 is greater in patients starting HAART at higher CD4 levels [Reference Garcia8–Reference Moore and Keruly10]. This therefore indirectly reflects the high prognostic value of the CD4 level reached on HAART [Reference Abrahamowicz and Mackenzie32]. Moreover, in the case of delayed HAART, the higher the baseline HIV viral load, the worse the outcome. This is consistent with the results of previous cohort studies [Reference Palella5, Reference Egger33], and probably reflects the poorer immunological outcome in cases of uncontrolled high level HIV replication in the absence of treatment. In contrast, when HAART is implemented immediately, the significance of baseline HIV viral load is outweighed by HIV viral load on treatment, and no longer influences the outcome [Reference Abrahamowicz and Mackenzie32].
Nevertheless, several limitations must be acknowledged. The study period ended in mid-2004, and the transitional probabilities may not accurately reflect the impact of current HAART, even though only currently recommended therapeutic schemes (i.e. HAART including two nucleoside inhibitors plus either a NNRTI or a PI boosted by ritonavir) were considered. The impact of a provider bias on transitional probabilities cannot be excluded either [Reference Palella5]. In addition to the ‘physiological’ fluctuation of the absolute CD4 count observed on HAART in patients with CD4 >500/mm3 [Reference Piroth25], fluctuations due to adherence, tolerance and resistance were not directly assessed. These are likely to explain why the final score for patients with CD4 >500/mm3 and immediately put on HAART was 6·4, far below the ideally expected duration of 10 years. This could also explain the mild gain in life expectancy observed in patients on immediate HAART in our study (3·3 years between CD4 <200/mm3 and ⩾350/mm3), since we considered that each year spent with a CD4 count <500/mm3 corresponded to 4–8 months with an optimal CD4 level (which is a difficult hypothesis). This gain in life expectancy is lower than that observed in a large cohort study (>7 years [34]), but within the range of another recent study (difference from 5·7 years to 0 between CD4 <200/mm3 and CD4 ⩾500/mm3 for a 40-year-old patient, depending on the level of adherence and HAART-related toxicity) [Reference Braithwaite35]. The differences in tolerance to, adherence to, and the virological efficacy of HAART probably explain these different gains. The increase in tolerance to HAART and its virological efficacy over time [Reference Kitahata18], in particular since the completion of our study, should, however, strengthen the benefits observed on HAART. On the other hand, it cannot be excluded that substantial sampling variability may have had an impact on the transitional probabilities, even though they are consistent with those observed in other cohorts. There is no statistical variability, since only the central estimation of the transitional probabilities (but not their distribution) is available when estimated from the Markov model. Thus only one estimate for each situation is used in the decision tree analysis. The only way to control this uncertainty was to perform representative sensitivity analyses of the different conceivable scenarios, as we did. Immediate HAART was always found to be beneficial in patients with CD4 <350/mm3 (data not shown).
It cannot also be excluded that 1-year transition may not be sufficient to capture important aspects of disease progression, in particular non-AIDS defining morbidity. Moreover, the outcomes did not directly include this non-AIDS-defining morbidity, of growing importance [Reference Emery11]. However, defining the preferred outcome as reaching and maintaining CD4 >500/mm3 in the main analysis indirectly took non-AIDS-defining morbidity into account, since this threshold was associated with a lower risk not only of AIDS but also of non-AIDS morbidity and mortality in untreated and treated patients [Reference Lewden7, Reference Kaufmann9, Reference Emery11, Reference Wang36]. It is important to point out that the complementary analysis focusing only on AIDS or death showed similar results and did not modify the direction and the magnitude of the differences observed.
In conclusion, this study, which relies on decision analyses that take into account not only immunological but also virological characteristics at baseline and real-life inputs, provides new data on the ‘when to start’ debate. While delayed HAART still appears to be the best approach in patients with a baseline CD4 count >350/mm3 and an HIV viral load <3·5 log10 copies/ml, immediate HAART is likely to be of interest in those with an HIV viral load >5 log10 copies/ml, with a slight preference towards boosted PI-based HAART. In those with a baseline HIV viral load between 3·5 and 5 log10 copies/ml, improvements in the management and virological efficacy of current antiretroviral drugs should favour immediate HAART. Even though these results are consistent with other recent findings [17, Reference Kitahata18], therapeutic trials are needed to help determine the best therapeutic approach for HIV-infected patients.
ACKNOWLEDGEMENTS
The authors thank Sandrine Vinault for her help in designing the decision tree, Catherine Lejeune for her valuable advice, and Philip Bastable for his help in reviewing the manuscript. The authors also thank all the physicians and the technicians involved in this study: Patricia Eglinger (Belfort); Christelle Braconnier, Benoit Broussolle, Romain Cailliod (Dijon); Marie-Pierre Bouillon, Mireille Stenzel (Nancy); Edith Ebel, Patricia Fischer (Strasbourg); Chantal Roche (Besançon); Philippe Choisy, Francis Marysse (Tourcoing).
DECLARATION OF INTEREST
None.









