INTRODUCTION
Cyclospora cayetanensis is an emerging infectious agent first described in individual patients with diarrhoea in 1977 [Reference Dawson1, Reference Ashford2] and then as a cause of foodborne outbreaks in the 1990s [Reference Shields and Olson3, Reference Herwaldt4]. This sporulating protozoan [Reference Shields and Olson3, Reference Ortega5] infects the upper part of the small intestine [Reference Shields and Olson3, Reference Eberhard and Arrowood6]. The symptoms of cyclosporosis include moderate to severe watery diarrhoea associated with abdominal pain, nausea, anorexia, weight loss and fatigue [Reference Dixon and Blais7]. The symptoms last longer in the very young or immunocompromised individuals [Reference Pape8, Reference Madico9]. The mean incubation period is 7 days, ranging from 1 to 14 days. The disease lasts from a few days to several weeks [Reference Erickson and Ortega10]. It may follow a relapsing remitting course in some individuals. The duration of symptoms, the risk of complication and the relapse rate are not well described in immunocompetent individuals in non-endemic countries.
From 1990 to 2005, most outbreaks in Canada and USA were associated with importation of contaminated food [Reference Shields and Olson3, Reference Herwaldt4]. Association with contaminated water was suspected in a few situations [Reference Huang11, Reference Karanis, Kourenti and Smith12]. Small fruits (raspberries, blackberries and others), mixed salad greens and herbs were involved [Reference Dixon and Blais7]. These products were presumably contaminated by contaminated surface water or by workers.
The parasite is excreted in stools in the form of oocysts that need days or weeks, depending on environmental conditions, to sporulate and become infectious [Reference Mota, Rauch and Edberg13]. Direct human-to-human transmission is thereby unlikely. Cooking is known to destroy the parasite, but not freezing [Reference Sathyanarayanan and Ortega14].
Cyclosporosis is considered endemic in many countries of South and Central America, in Africa and in Asia [Reference Shields and Olson3]. Diagnosis is based on microscopic examination with appropriate staining techniques (modified acid-fast or modified safranin), demonstration of oocyst autofluorescence by UV fluorescence microscopy, demonstration of sporulation or molecular methods [Reference Shlim15, Reference Botero-Garces16].
This study describes an outbreak involving 250 adults exposed to contaminated food. It focuses on the duration and the relapse of symptoms, the occurrence of complications and evidence of local transmission.
METHOD
Context
This outbreak occurred in seven groups of workers who ate in a restaurant in June 2005 in the region of Monteregie (Quebec province, Canada). This region has a population of 1 415 000 inhabitants (127·4 persons/km2). Each group worked in a different location and had no other activities in common. A special menu was prepared for the occasion and offered to all groups: fruit cocktail, tomato and bocconcini cheese appetizer, a choice of three entrees (salmon, poultry, pork) served with cooked vegetables, chocolate cake with fresh fruits, tea, coffee and water. The contaminated food was taken at lunch or dinner by 94 and 156 individuals on 29 and 30 June, respectively. A total of 247 adults from the seven groups and three restaurant employees were exposed.
The first cases were reported to the regional Public Health Department on 9 July. An outbreak investigation was launched and included case interview, pathogen identification from stool samples, transmission of information to physicians and affected individuals. The provincial and federal food inspection services [Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ); Canadian Food Inspection Agency (CFIA)] were notified and they started their own investigation. In the province of Quebec, federal-provincial agreements define their respective jurisdiction. This investigation included inspection of the restaurant premises, review of the menu, trace-back activities, verification of the health status of food handlers and of the provincial distribution network for these products and food recall activities.
Data collection
Data on affected people were collected from three sources. The first source was the public health files completed during the outbreak investigation. The lists of exposed people were obtained from the person in charge of each group. Thirteen public health professionals tried to contact all exposed individuals, completing an epidemiological questionnaire by telephone interview between 10 and 25 July 2005. Patient identification, symptoms (i.e. diarrhoea, nausea, weakness, dizzy spells, abdominal cramps, fever, headache, vomiting, bloody stools), medical consultation, laboratory results and consumption of food items were reviewed.
The second source was a follow-up survey performed 3 months after the outbreak onset. Questionnaires were sent by mail to all symptomatic individuals with a known address even if they did not meet the case definition. To increase the response rate, questionnaires were answered anonymously and returned in prepaid envelopes. This questionnaire reviewed sociodemographic data, medical consultation and hospitalization, treatment and evidence of local transmission. The duration of diarrhoea and fatigue were recorded for the period running from 1 July to 30 September 2005.
The third source was medical charts of patients with persisting symptoms who consulted an infectious diseases clinic (McGill Center for Tropical Diseases). These were reviewed in August 2007.
Stool cultures for Campylobacter, Salmonella, Shigella and Yersinia were performed using standard procedures; MacConkey sorbitol agar medium was used for E. coli O157. Examination for viral infection was not done. Stool samples were concentrated by formalin-ethyl acetate sedimentation and examined for parasites and oocysts of Cyclospora sp. under 1% iodine preparation or modified acid-fast staining. Most parasite identifications were cross-checked by the Laboratoire de santé publique du Québec, the provincial reference laboratory.
A case of cyclosporosis was defined as onset of illness 1–14 days after consumption of food or beverages served at the restaurant on 29 or 30 June. Confirmed cases had Cyclospora sp. identified in a stool sample and at least one gastrointestinal (i.e. diarrhoea, nausea, vomiting, abdominal cramps, loss of appetite, unintentional weight loss) or constitutional (i.e. fever, headache, fatigue) symptom. Probable cases either had diarrhoea and at least one other symptom or five or more symptoms, including at least three gastrointestinal symptoms. Diarrhoea was defined as three or more loose or liquid stools per day [Reference Christ17].
Statistical analyses
A retrospective cohort study was performed to see if any food item was associated with disease. Relative risks (RR) and 95% confidence intervals (CI) were computed. Relapses were studied for two different symptoms: diarrhoea and fatigue. They were defined as recurrence of symptoms after a 3-day asymptomatic period. When appropriate, proportions were compared with χ2 test and means with Student's t test. The level of significance for all analyses was P<0·05.
Ethical aspects
Cyclospora infection is a notifiable disease in Quebec. Therefore, this study was conducted as part of regular public health activities. No ethical board review was performed. Denominalized data was used in all analyses.
RESULTS
Outbreak investigation
A total of 142 cases were identified. Table 1 shows the number of files reviewed from each source. Six symptomatic individuals could not be classified as probable cases because they had between two and four symptoms but no diarrhoea.
Table 1. Response rate and classification of respondents obtained from each source
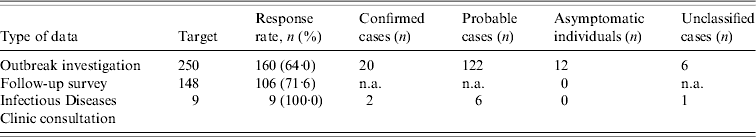
n.a., Not available because information required to classify cases was not collected.
Table 2 shows the distribution of symptoms in this outbreak compared to those reported in 760 laboratory-confirmed cases caused by imported raspberries in 1996 [Reference Herwaldt18]. The most dominant symptoms were abundant aqueous diarrhoea with nausea, abdominal cramps and fatigue. Fever and vomiting were reported at the beginning of the illness. Headaches were generally not severe. The distributions of symptoms were the same for confirmed and probable cases (P>0·05, data not shown). The attack rate was estimated at 89%.
Table 2. Sociodemographic data and symptoms of cases and comparison with another outbreak
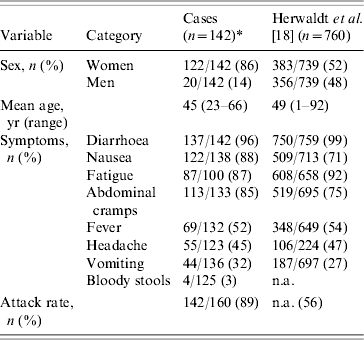
n.a., Not available.
* The denominators for the symptoms vary because of incomplete data.
The epidemic curve is shown on Figure 1. The symptoms started on average 7·3 days after exposure (median 7 days). For the purpose of the investigation, 20 individuals from six of the seven groups were asked to submit a stool sample for analyses. Cultures for Campylobacter, Salmonella, Shigella, Yersinia and E. coli O157 were negative. Stool samples were positive for Cyclospora sp. in the 20 individuals. In two of them, parasites of controversial pathogenicity (Dientamoeba fragilis or Blastocystis hominis) were also identified. All samples were tested within 3 weeks following the onset of symptoms.
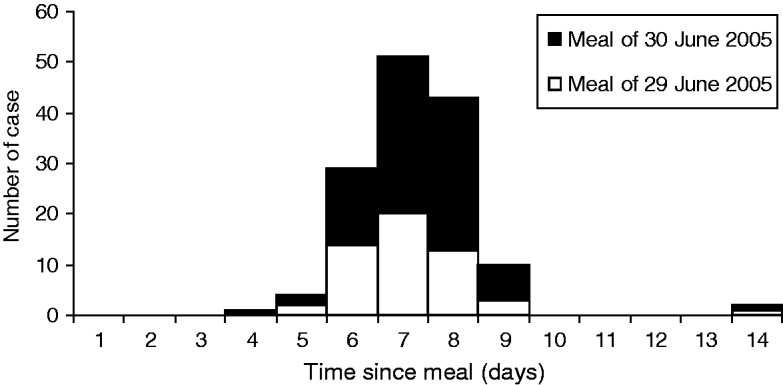
Fig. 1. Case distribution according to the delay between meal and onset of symptoms (n=140).
The epidemiological investigation showed that the tomato and bocconcini cheese appetizer was associated with disease. In a retrospective cohort study, 94% (133/141) of those exposed to the appetizer and 0% of those unexposed (0/4) were sick (RR undefined, Fisher's exact test P<0·0001). There were no significant differences between exposed and unexposed individuals for other food items (RR between 0·9 and 1·1), including the chocolate cake with fresh fruits (RR 1·1, 95% CI 0·8–1·5, Fisher's exact test P=0·48). The investigation by MAPAQ and CFIA concluded that this outbreak was most likely due to fresh basil imported from Mexico and delivered to the restaurant on 28 June 2005. This fresh basil was used to prepare the uncooked appetizer. Testing of subsequent lots of basil originating from the same farm was not conclusive.
Follow-up study
The confirmed, probable and unclassified cases were all included in the follow-up study. However, we did not collect the information required to classify those who returned the questionnaire anonymously. Demographic and clinical characteristics of respondents are shown in Table 3. Symptoms appeared to last longer in those who took antibiotics. The mean duration of diarrhoea for people who took antibiotics was 14·8 days compared to 9·7 days in those who did not (P=0·11). The mean duration of fatigue was 31·5 days for people who took antibiotics compared to 23·1 days for those who did not (P=0·08).
Table 3. Demographic and clinical characteristics of respondents to the follow-up study
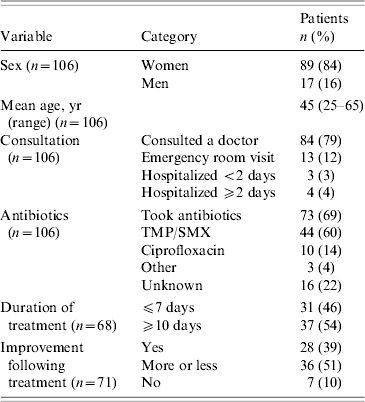
TMP/SMX, Trimethoprim-sulfamethoxazole.
For 100 respondents, the mean duration of diarrhoea was 14 days [interquartile range (IQR) 4–18; maximum 88]. Twenty-five had relapses of diarrhoea (mean of two relapses). Of 102 respondents, the mean duration of fatigue was 30 days (IQR 11–42, maximum 92). Ten had relapses of fatigue, often described as a feeling of exhaustion and the inability to perform daily activities (mean of two relapses). On 30 September, two respondents still presented episodes of diarrhoea and 11 still experienced excessive fatigue.
There were at least five pregnant women in the affected individuals and no particular problems were noted for them. One case had a family and a personal history of inflammatory bowel disease at the time of the outbreak. Despite antibiotic treatment, a partial resection of the colon had to be performed. There were no other complications or any death. Only one case could be due to local transmission of the parasite: a man unexposed to the contaminated meal and whose wife had profuse diarrhoea and severe fatigue. He developed compatible symptoms 6 days after her but he did not have a medical consultation or a stool examination.
Infectious Diseases Clinic consultation
Consultations at the infectious diseases clinic occurred in autumn 2005 except for one person who was followed in 2006. There were eight women and one man aged from 25 to 58 years. The information recorded in the medical charts was similar to that available in the public health files. Laboratory studies conducted at this clinic for seven persons were negative for Cyclospora sp. and other pathogenic agents. No specific treatment was prescribed. For those cases with lasting symptoms, the suspected diagnosis was post-infectious irritable bowel syndrome (IBS).
DISCUSSION
This outbreak affected seven groups of workers who attended the same restaurant in June 2005. The clinical presentation, incubation period and presence of Cyclospora sp. in all stool samples tested point to C. cayetanensis as the cause of this outbreak. The investigation identified contaminated basil imported from Mexico as the source. All the remaining basil was recalled by the Mexican supplier which led to subsequent recalls involving distributors, processors, retailers and restaurants. These recalls targeted all the basil received from the end of June to the end of July. Afterwards, basil importation from this farm was suspended. Two other outbreaks, in 1999 and 2007, were also attributed to fresh basil of Mexican or American origin [Reference Lopez and Dodson19, Reference Shah20]. Although a mean of 12 confirmed cases of cyclosporosis are reported in Quebec each year, no other case associated with fresh basil was identified that year. This may be due to the fact that reported cases are usually travel-related and clinicians have a low index of suspicion and do not look for Cyclospora sp. in locally acquired diarrhoea. Moreover, an important part of the contaminated batch was transformed in a cooked product, like soup, a process which eliminates the parasite. It also seems likely that only a part of the field had been contaminated by a sick picker or irrigation water.
The epidemiological confirmation of the source was difficult due to the small number of unaffected individuals. High attack rates have also been observed in other outbreaks which occurred in Canada and USA [Reference Shields and Olson3, Reference Herwaldt4]. However, some exposed individuals may be less symptomatic or asymptomatic because of the small dose ingested or the individuals' resistance [Reference Eberhard and Arrowood6, Reference Erickson and Ortega10]. Stool analyses performed early in the course of the disease were positive for Cyclospora sp. while analyses performed later were negative, confirming the necessity of early investigation [Reference Blans21].
Diarrhoea and fatigue were common in this outbreak. The frequency of the symptoms observed was similar to that observed in other cluster-related cases in Canada and USA [Reference Herwaldt18]. Conversely, these symptoms were less common in an endemic country like Peru [Reference Alva22] where diarrhoea, abdominal cramps and fever were reported in 70%, 60% and 19% of cases, respectively. A proportion of the Peruvian population must be partially immune to Cyclospora which would explain those observations. The long duration of symptoms should be noted. In half of the cases, diarrhoea lasted for ⩾9 days and fatigue for ⩾21 days. Unfortunately, articles describing outbreak investigations rarely report on the severity and duration of symptoms.
Human-to-human transmission of this parasite is unlikely since it needs sporulation in the environment to become infectious [Reference Erickson and Ortega10]. The follow-up study performed in a non-endemic setting enabled us to confirm that environmental conditions in Quebec are unfavourable to parasite maintenance. Indeed, even though many cases had young children at home, only one possible (but unconfirmed) case of local transmission could be found during our investigation.
The usefulness of the antibiotic treatment was not demonstrated in this study, unlike previous ones [Reference Madico9, Reference Hoge23, Reference Yazar, Yalcln and Sahin24]. Furthermore, our results suggest that those who took antibiotics were sicker and had symptoms of longer duration than those who did not. Indeed, people with more symptoms may be more inclined to seek treatment.
IBS is a functional bowel disorder characterized by chronic abdominal pain, discomfort, bloating, and alteration of bowel habits in the absence of any detectable organic cause [Reference Connor25]. In post-infectious irritable bowel syndrome (PI-IBS), these symptoms follow an episode of enteric infection which is characterized by fever, diarrhoea and vomiting. The proportion of patients with enteric infection who have PI-IBS ranges from 3% to 36%. Risks factors include younger age, severe disease and longer duration of the initial episode [Reference Connor25]. This syndrome is more often reported after bacterial infections, but because of its protracted course Cyclospora infection would be a good candidate.
Selection and information bias could have affected our results. Sixty-four percent of the exposed individuals were reached during the initial investigation and 72% of those targeted returned the follow-up questionnaire. A report showed that the involved worker population comprised 85% of women and had an average age of 39 years [26], which is quite similar to what was observed in our study. Information obtained from persons in charge of each group suggested that most individuals that could not be interviewed were indeed symptomatic. So, overestimation of the attack rate is unlikely. However, in the follow-up study, sicker individuals could have been more likely to return the questionnaire than healthier ones. Recall bias was minimized because description of symptoms and food history were obtained within the 3 weeks following the disease onset by experienced public health professionals working in the same office.
CONCLUSION
Outbreaks of cyclosporosis continue to occur in non-endemic European and American countries showing the effect of globalization of food supply as well as the increasing importance of fresh produce as vehicles for foodborne outbreaks [Reference Shah20, Reference Insulander27].
In a naive population of immunocompetent adults, Cyclospora infection presents with watery diarrhoea lasting from 4 to 18 days and causing fatigue lasting from 11 to 42 days. For a small proportion of individuals, recovery can be delayed. Being relatively unknown in developed countries, sporadic cases may be overlooked. We suggest that in future outbreaks, a letter explaining the disease, its diagnosis and treatment, should be sent to clinicians and health hotlines. This was done successfully in this outbreak and was welcome.
ACKNOWLEDGEMENTS
This study was funded through regular public health activities. For their valuable help we thank Dr Hugo Hébert, Thèrèse Monast, Nicole Trudeau and other physicians and nurses working at the Monteregie Public Health Department. We also thank the personnel of the Direction générale de la santé animale et de l'inspection des aliments of the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ) and the Canadian Food Inspection Agency who participated in this investigation. We thank the late Dr John D. Maclean from McGill Center for Tropical Diseases for his advice and support. Last, we thank the laboratory staff from the Haut-Richelieu Hospital and the Laboratoire de santé publique du Québec who confirmed the cause of this outbreak.






