INTRODUCTION
Legionella can cause legionellosis which includes two disease forms: a self-limiting flu-like illness called Pontiac fever or a more severe, potentially fatal, pneumonia called Legionnaires’ disease. Annually, 8000–18 000 people are hospitalized with legionellosis in the USA [Reference Marston1]. Since the reporting of disease outbreaks due to Legionella began in 2001, Legionella has been shown to cause more drinking-water-related outbreaks than any other microorganism [Reference LeChevallier and Edzwald2].
Legionella species are naturally occurring and ubiquitous in the environment and are most likely to reproduce in high numbers in warm, stagnant water. These bacteria have been identified in many different kinds of water and water systems, such as hot- and cold-water taps and showers, creeks, ponds, whirlpool spas, cooling towers and evaporative condensers of large air-conditioning systems [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3]. L. pneumophila is the most common aetiological agent of legionellosis [Reference Yu4]. The primary human exposure route to Legionella is the inhalation of aerosolized water containing high concentrations of the microorganism. Older adults, smokers, individuals with immunocompromised conditions and comorbidities are at higher risk of legionellosis [Reference Silk5]. Incidence of legionellosis is greatest during the summer and autumn months [Reference Neil and Berkelman6].
The global incident rate of legionellosis is not clear since there is no standard identification and reporting mechanism; however, there was a reported US national incidence rate of 1·15 cases/100 000 persons in 2009 [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3, 7]. The case-fatality rate of legionellosis ranges from 1% to over 15% with a higher fatality rate observed in hospital-acquired infections compared to community-acquired infections [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3, Reference Bull8, Reference Benin, Benson and Besser9]. Community outbreaks of legionellosis have been linked to decorative fountains, humidifiers, respiratory therapy devices, and misters (such as those found in the produce section of grocery stores), and most often cooling towers [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3, Reference Bull8].
Key factors involved in microbial growth within a distribution system include ineffective disinfection residuals, temperature and availability of nutrients [Reference LeChevallier and Edzwald2]. Legionella typically live as intracellular parasites of protozoan hosts, which become embedded within disinfectant-resistant biofilms inside water pipes [Reference Declerck10–Reference Thomas13]. Free-living amoebae, a Legionella host, are consistently detected in treated drinking-water systems and are capable of breaking through water-treatment barriers and entering distribution systems [Reference Thomas and Ashbolt12, Reference Pryor14]. Sufficient control of Legionella requires a constant disinfectant residual throughout the water system [Reference Thomas13]. Control of Legionella requires at least 0·2 mg/l [equivalent to parts per million (ppm)] free residual chlorine to cause stasis of growth and at least 1–2 mg/l to kill [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3, 15]. However, the protection afforded by biofilm and amoebae means that achieving a several log reduction in the number of microorganisms requires at least 50 mg/l [Reference Kilvington and Price16, Reference Kuchta17]. High levels of sediment in water mains may consume free chlorine. Sedimentation largely originates in particulate material that escapes the treatment plant, bits of torn biofilm fed by soluble nutrients not removed in the treatment plant and rust particles from mains, as well as by growth in water pipe segments experiencing low water flow.
Temperature plays an important role in the colonization of Legionella in water systems [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3]. Risk of legionellosis is increased by presence of Legionella in water and warm temperatures which encourages proliferation [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3]. The optimal temperature range for Legionella growth is in the 35–45 °C range, but the organism has been shown to withstand temperatures of 50 °C for several hours. It does not multiply below 20 °C [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3]. Additionally, microbial growth requires adequate nutrients, particularly organic matter, which is typically present in greater quantity in source waters reliant on surface water rather than groundwater sources and higher levels can result in the increase of Legionella concentrations [Reference LeChevallier and Edzwald2, Reference Wullings, Bakker and van der Kooij18]. During 2006 and 2007, the New Jersey Department of Health (NJDOH) collaborated with a local health department to investigate two legionellosis pneumonia outbreaks, one at a geriatric centre and the other in high-rise housing for seniors located a short distance away. During these investigations it was observed that additional cases occurred close by, but in smaller residential settings, during the years 2003–2007. This apparent geographical cluster of legionellosis occurred in the same general area of a community water system storage tank. We undertook an investigation to determine the association of the water storage tank and corresponding distribution system and this putative cluster. In addition we describe the role the investigation played when cases of legionellosis began recurring in the investigation area.
METHODS
Case identification
Legionellosis is a nationally notifiable disease in the USA and many other countries. Laboratory confirmation of legionellosis is required to be sent to local health departments within 24 h of diagnosis and local health departments subsequently report cases to the state health department. Legionellosis is confirmed when a case meets one of the following laboratory diagnostic criteria: by culture isolation of any Legionella organism, by detection of specific L. pneumophila serogroup 1 antigen in urine, or by seroconversion criteria. Like other state health departments NJDOH maintains a web-based Communicable Disease Reporting Surveillance System (CDRSS) of all confirmed and suspected cases of legionellosis and frequently collaborates with local health departments to investigate possible outbreaks. All confirmed cases of legionellosis from 2003 to 2007 were extracted from CDRSS.
Initial investigation (2003–2007)
The investigation of four cases of legionellosis at a high-rise seniors’ housing building initially focused on the interior plumbing of the building. Investigators conducted environmental sampling for Legionella, all water samples were sent to a laboratory certified to culture Legionella which used standard Centers for Disease Control and Prevention (CDC) guidelines [19]. Neither cold- nor hot-water sampling revealed Legionella but sampling showed high bacterial counts of both Gram-positive and -negative bacteria. Additionally cases were detected at a geriatric centre and environmental sampling detected positive samples for Legionella as well as high bacterial counts of Gram-negative and -positive bacteria. Subsequently it became clear that there were other cases in the area, including persons living in single-family housing. All cases were confirmed by a urinary antigen assay for serotype 1. All cases were surveyed concerning environmental water source exposures and no common link was identified. Cases were a mixture of both transient and non-transient, some of which were homebound and never left their respective facility. The recurrence of cases both outbreak-associated and sporadic occurring in the same adjacent area spurred additional investigation of a possible underlying source of Legionella in common among these case locations.
In collaboration with local health staff, investigators performed ground searches and examined web-based satellite images from Google Earth. Searches did not reveal a cooling tower or aerosol-dispersing device which may have been the potential source of Legionella exposure. Without identification of a common source of exposure investigators examined other possible potable water sources of Legionella. As cases appeared to be clustering around a water storage tank further investigation was made into the tank and surrounding distribution system.
The community water system provided its 2005–2007 free chlorine residual data from total coliform sample sites. The data indicated chlorine residual levels below the recommended 0·2 mg/l at total coliform sample sites as a regular feature of warm weather water quality near the tank. Low chlorine residual levels suggested that either the tank was compromising water quality in the area or that a high level of sediment in local mains was consuming the free chlorine. Additionally investigators reviewed results from standard monitoring of total coliforms which requires the water system to collect a minimum 120 samples per month throughout the distribution system. Although there were no data on the water temperature in the mains, the surface-water source had average daily temperatures in the 25–35 °C range during July and August, according to United States Geological Survey (USGS) monitoring data [20]. The water system servicing this area relied on a treated surface-water source, which has been shown to have higher concentrations of Legionella than found in groundwater sources and usually has higher levels of organic matter than groundwater [Reference Wullings and van der Kooij21]. This preliminary information suggested that potential sources of Legionella were overgrowth in the tank and/or in the distribution system mains in the area, and ultimately in the residential indoor plumbing.
Investigation areas
Inadequate disinfection of water in the storage tank could subsequently result in low chlorine residual levels throughout the distribution system, especially with increasing distance from the storage tank. Therefore, we estimated the extent of impact the water storage tank posed on surrounding areas in order to develop an investigation area. Without data on distribution flow from the storage tank we used two methods to define an investigation area based on chlorine residual sampling values from nearby parts of the distribution system available from 2005–2007 for warm-weather months. For the first method we defined investigation area A by drawing an ~1 mile circle around the tank, modified by the presence of a water system boundary in the area, as well as the paucity of water mains crossing a local highway, a local park, and a local creek (Fig. 1a ). It was assumed that the large mains supplying the area passed down the main streets. The 1 mile circle was chosen in part because two cases in the initial investigation resided at a geriatric centre ~1 mile in distance. The population in that area was ~9000, which would be consistent with the area of influence of a 1 million gallon water tower, based on the assumption that the water system cycled the water in the tank on a daily basis and that the average per person water use is estimated to be in the 100 gallons/day range [22].

Fig. 1. Estimate of census blocks within an estimated area of influence of a water storage tank estimated by two independent methods, with overlay of case counts, 2003–2007. (a) Investigation area A; (b) investigation area B.
We chose a second method for delineating the area of influence of the tank which did not include any case location information or specific knowledge of the distribution system around the tank. We created 1000-ft buffers around the water storage tank using ArcGIS and averaged chlorine residuals from each sample site within a given buffer for the creation of investigation area B. We considered the potential area of influence of the tank to extend to the buffer furthest away from the tank for which chlorine residual data were available and had levels below the recommended 0·2 mg/l. The area of influence of the water storage tank was bounded by the larger water system boundary and included census block population data for those blocks in which substantial area was overlapped by the buffer (Fig. 1 b). Three-year average chlorine residual levels for each month for samples in each buffer ring were calculated to illustrate whether there was a warm-weather pattern and whether chlorine residual levels tapered by distance from the storage tank.
Data analysis
We used SaTScan™ (http://www.satscan.org/) to determine whether there were any statistically significant geographical clusters of legionellosis from 2003 to 2007 in the state, adjusted for age and gender. Multiple scans were performed whereby the maximum percentage of total population at risk was varied from 1% to 10% by 1% increments as well as the default 50% [Reference Chen23, Reference Kulldorff24]. The geographical unit of analysis was census tract. We used U.S. Census Bureau 2000 population data.
We compared mean and range of ages as well as gender distribution of the cases in the two investigation areas, the area serviced by the water utility, by the county, and the state. The water utility is inclusive of the investigation areas. Annual average incidence rates of legionellosis/100 000 residents during 2003–2007 in the investigation area and in the entire water system service area were calculated. Additional rate calculations were performed to further characterize legionellosis incidence in the county and the state. All rates were further restricted to those aged ⩾50 years because of the increased risk of legionellosis in older adults.
RESULTS
Findings from the initial investigation (2003–2007)
Findings from the SaTScan™ spatial cluster analysis indicated 3–4 statistically significant clusters of age- and gender-adjusted legionellosis in the state when using different maximum percentage of total population at risk cut-offs. In all scans the primary cluster identified (P < 0·001) consisted of four census tracts which included the water storage tank and corresponding investigation area.
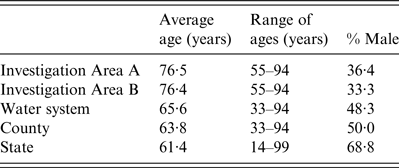
Statewide during 2003–2007, there were 512 confirmed cases of legionellosis. Investigation area A included 11 cases while investigation area B included nine cases (Fig. 1a, b ). Cases located in both investigation areas were all aged ⩾50 years (Table 1). The average age of cases in the state was 61 years whereas the average age was 76 years in cases residing in the investigation area, both A and B (Table 1). The percentage of confirmed cases that were male was smaller in the investigation area (A and B) compared to the state. Figure 2 illustrates 3-year average (2005–2007), chlorine residual sampling for warm-weather months (May–October), by buffer distance from the water storage tank. The lowest chlorine residuals were seen either in the 3000 ft or 4000 ft buffer areas for all months. Regulatory total coliform sampling throughout the entire distribution system detected two positives, one in May 2004 and the other in August 2005, follow-up testing returned negative results. Faecal coliforms and E. coli were not found.
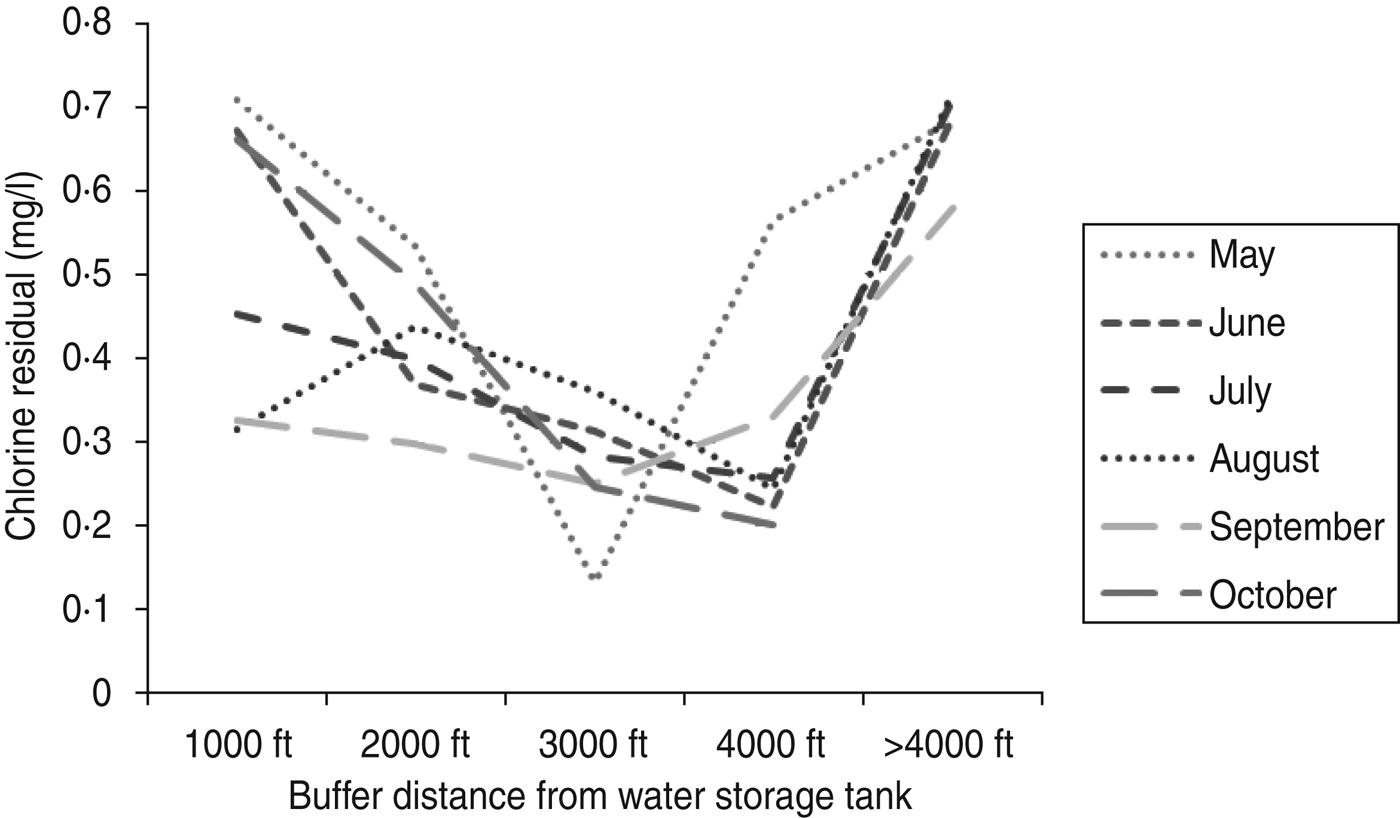
Fig. 2. Three-year (2005–2007) average chlorine residual levels (mg/l) for warm-weather months by buffer distance from water storage tank.
Table 1. Average age, range of ages and percentage of male of cases in the investigation areas, water system, county and New Jersey, 2003–2007
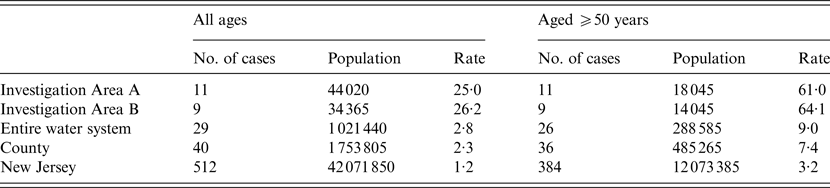
We compared rates of legionellosis (2003–2007), in the state, the county, the area serviced by the water system, and the two investigation areas (Table 2). Among all ages, the rate of legionellosis in investigation area A was 25·0/100 000 residents and in investigation area B it was 26·0/100 000 residents. These rates compare unfavourably to the rest of the water system with a rate of 2·8/100 000 residents. Further, the rate of legionellosis in the county was 2·3/100 000 residents and 1·2/100 000 residents in the state which highlights an inflated case rate in the investigation area. The increased rate in the investigation area is further inflated compared to the other geographical areas when the rate population is restricted to those aged ⩾50 years. In investigation areas A and B, the rate of legionellosis was 61·0 and 64·1/100 000 residents aged ⩾50 years, respectively, about eight times larger than the rest of the water system (9·0/100 000 residents aged ⩾50 years) and almost 20 times larger than the state rate (3·2/100 000 residents aged ⩾50 years).
Table 2. Annual average incidence rate of legionellosis per 100 000 residents among all ages and those aged ⩾50 years by investigation area, entire water system area, county and New Jersey, 2003–2007
Findings during 2012
Our results from the initial investigation (2003–2007), strongly suggested a need for public health action and interventions involving the water utility; however, our monitoring of the area continued throughout 2008–2009 and no additional cases were observed. It was incorrectly assumed that the issue had resolved itself. Free chlorine levels were still low during 2008–2009, but especially low, with five instances of chlorine residuals falling below 0·05 ppm, during the particularly hot summer of 2010. During the warm months of 2010 we began to see a similar pattern of case increases in both larger institutional settings and smaller residential settings. This increase of legionellosis in conjunction with findings from our 2003–2007 analysis, which suggested the involvement of the water storage tank and respective distribution system in this area, strongly indicated that action should be taken.
A contractor for the water system performed a distribution system hydraulic analysis involving staff interviews and a partial assessment of water quality in the storage tank. Results from the distribution system hydraulic analysis indicated that water in the tank was stagnant and that the investigation area was predicted to have low flow. Interview of water system staff indicated that regular flushing of water mains had not been conducted in years, and probably never.
A programme of unidirectional flushing water mains in the area including Legionella testing was performed by a contractor for the water utility in 2011 and was mostly complete by the end of 2012. The flushing programme included mains in five of six of the residential streets where cases were located. Among the 56 L. pneumophila samples taken from 12 sites located within the targeted flushing zone, L. pneumophila serogroup 1 (1–2100 c.f.u./25·2 ml) was found in 25% of the sample sites from pre-flushing samples, 50% of the sample sites from flushing samples, and one location had a post-flush detection. The latter street had to be flushed a second time because Legionella was found in the first post-flushing samples. Another street had been flushed 5 months prior to the Legionella testing, and no Legionella was found before, during, or after the second flushing event.
Four of 53 samples taken during the sampling for Legionella detected total coliforms (maximum of 18/100 ml), but none were faecal coliform positive (one sample had confluent total coliforms, and so had questionable validity). Heterotrophic plate count (HPC) testing during the sampling for Legionella found three instances of HPC at > 100/ml and five instances of HPC at 10–99/ml out of 53 samples. This was the first time the residential mains in the area had been tested.
The community water system storage tank was drained, washed out, and disinfected in spring 2012. Depending on the outcome of an evaluation project, the storage tanks in the water system will undergo a project to promote water mixing and reduce water age. Notably, free chlorine levels in the area returned to a healthy level in 2012. Other projects include an ongoing 5-year distribution system cleaning and cement mortar lining, evaluation of improving circulation in the distribution system and evaluation of improvements to the treatment system.
DISCUSSION
The legionellosis outbreak appeared to be community-based because cases occurred in both outbreak-associated larger buildings and sporadically in smaller, single-family residential settings. Cluster analyses confirmed that there was a statistically significant geographical cluster of legionellosis involving four census tracts. Investigation of the area did not reveal a cooling tower or other aerosol-dispersing devices. Further investigation suggested that the origin of the outbreak could be the community water system infrastructure in the area since conditions for Legionella growth were present including low chlorine residual levels in local water mains during warm months, stagnant water in the storage tank, and no flushing programme to clear sediment from water mains. The conditions for Legionella growth in the water distribution system mains were consistent with the ready detection of Legionella in mains on residential streets during a recently conducted flushing programme in the area.
The rate of legionellosis due to L. pneumophila in residents aged ⩾50 years in the investigation areas was eight times higher than in the service area and almost 20 times higher than the overall rate in New Jersey. Subsequently, L. pneumophila was found in water mains sampled near case residences Although other outbreak environmental investigation(s) sampled public water system as a potential source of disease, to the best of our knowledge a community water distribution system has never previously been implicated as the source of a community-wide outbreak of legionellosis [25–Reference Rota27].
We used two independent approaches to define the investigation area. The first method to create investigation area A utilized information about pipelines, outbreak investigation area, chlorine residual sampling, and population served by the tank. The second method to create investigation area B used buffered distance and chlorine residual sampling. We chose to present findings from the two independent methods as a sensitivity analysis for rate calculations. Investigation area A included two additional cases and a slightly larger population base. These two cases were located very close to investigation area B during the 2003–2007 study period but were not captured, suggesting that this estimate of the extent of influence of the water storage tank was conservative. Additionally, two more cases were located just outside of both of the investigation areas which may belong in the investigation, but were left out due to limited chlorine residual sampling information. Without detailed information on water distribution, flow, and system pipelines we were limited in our ability to accurately assess the true area of influence of the water storage tank and corresponding distribution system. While a search of the area did not reveal a cooling tower, it is still possible that one existed in the area.
Our analysis was limited since the identification of cases is based on positive urine antigen for L. pneumophila serogroup 1, which accounts for 70–80% of legionellosis in the USA, therefore pneumonia caused by other serotypes or species were not included [Reference Fields, Benson and Besser28]. The culture method used by the water system contractor did not provide a serotype. The environmental investigation was limited without clinical isolates to confirm that Legionella detected in water mains was of the same serotype as the urine antigen assay detected in cases. A comparison of disease rates, pre- and post-intervention, would enhance the conclusion of an association of a community-outbreak of legionellosis with the community water distribution system. However, interventions in our investigation took place over a period of years, primarily 2008–2012 and many efforts still continue. A report on the effect of the intervention would be several years off.
Free residual chlorine levels were chronically low in the investigation area during warm-weather months. Current regulations require drinking-water providers to maintain detectable chlorine residuals of at least 0·05 mg/l in the distribution system for the control of faecal coliforms and E. coli. However Legionella is much less sensitive to a low level of free chlorine than faecal coliforms or E. coli. Environmental samples taken from two buildings involved in the community cluster had high bacterial counts, indicating a low level of protection afforded from the disinfectant residual available inside the building. While there are no specific federal, industry or World Health Organization (WHO) regulations or guidelines on chlorine residual levels to control Legionella growth in the water distribution system, the scientific literature and advice from WHO suggests that a minimum of 0·2 mg/l free residual chlorine be maintained in the water system for primary control of Legionella and that much higher concentrations (up to 50 mg/l) are required to kill the organisms residing inside biofilm [Reference Bartram, Chartier, Lee, Pond and Surman-Lee3]. Although regulation of Legionella has been in development for years no drinking-water regulation specific to Legionella exists; however, it is believed that the treatment requirements for the removal or inactivation of Giardia lamblia will control Legionella [29]. Although information on water temperature in the mains was not available, the water source had average daily summer temperatures in the 25–35 °C range [20]. The water was probably heated further in the storage tank located in the area. Cases occurred in late summer–early autumn, a time at which cumulative Legionella growth would be expected to peak in a water system.
Subsequent hydraulic analysis conducted by the water system indicated that low-flow conditions existed within the distribution system area, allowing for sediment build-up in the mains increasing consumption of the chlorine residual. The water system showed that flushing had not been conducted for many years. The U.S. Environmental Protection Agency (USEPA) guideline calls for a regular flushing of the mains, especially in low-flow areas [30].
Cases occurred sporadically. The episodic nature of the cases is consistent with the sloughing of biofilm during pressure shocks that occur in water mains due to valve openings or closures or due to high shear during high demand, such as a fire-fighting [Reference LeChevallier31]. This is consistent with the detection of Legionella in only 25% of mains before flushing, while 50% of the sample sites detected Legionella during flushing. The sporadic pattern of biofilm release even during flushing indicates that future investigators should be prepared to test many samples. Additionally, hydraulic analysis indicated there was little mixing of water in the storage tank, but it is possible that occasional hydraulic events, like fire-fighting, caused the movement of stagnant tank water into the local distribution system, helping to keep the system seeded with Legionella.
There were six drinking-water outbreaks in the USA due to Legionella during 2001–2002, all of which occurred in large buildings and institutions and were associated with the respective plumbing systems in these buildings [Reference LeChevallier and Edzwald2, Reference Hlavsa32]. Deficiencies occurring in plumbing and pipes inside buildings are not the responsibility of the water utility [Reference Liang33]. However, water distribution systems have been shown to play a role in the transmission of Legionella and may contaminate the plumbing systems of buildings [Reference Lin34, Reference States35]. Some water systems have switched to chloramines to better control biofilms. Buildings supplied by municipal water systems which have switched to monochloramines have shown marked reduction of Legionella colonization [Reference Moore36, Reference Flannery37]. Further, hospitals served by public water systems using chloramines reported fewer outbreaks of legionellosis than those using free chlorine [Reference Kool, Carpenter and Fields38, Reference Heffelfinger39]. These studies highlight the potential impact community water system distribution system biofilm control has on the transmission of disease and control of Legionella.
Investigators are tasked with the challenge of identifying sources of Legionella exposure when outbreaks and clusters are identified. Investigations often lead to varying degrees of certainty of source identification and often no source is ever identified. After statistical analysis, methods were used to confirm that case increases in the identified area were not likely to have occurred by chance alone and investigators took further action. An aerosol-dispersing device, like a cooling tower, or other environmental source was not identified to epidemiologically link cases in the community outbreak, especially since some cases were confined to indoors. Subsequent inspection into the public water distribution system revealed poor maintenance leading to conditions which could support the growth of Legionella. Consequent sampling revealed Legionella in the water mains where cases resided. In the absence of case interviews and environmental sampling linking cases to a common source this evidence was compelling and investigators worked with the community water supplier to implement an intervention. There still remains uncertainty as to the common source.
In summary, this study suggests that there is a need to update and expand the standard dogma, that water heaters, indoor plumbing, and source water are the reservoirs of Legionella, while community outbreaks require an air-dispersal source. Public health investigators should not exclude the community water system from consideration as the disease transmission vector, especially when a standard common source is not found. The maintenance and disinfection potential in water distribution mains must also be included, particularly in community water systems supplied by surface water, where summer temperatures and nutrient levels can create conditions conducive for the amplification of Legionella growth in biofilms. Water utility and regulatory authorities should take actions when low chlorine residuals are identified during the hot summer months, regardless of results of total coliform tests. Public health action in the absence of disease is recommended. Low chlorine residuals can be improved by flushing of mains, checking for closed valves that can result in hydraulic ‘dead-ends’, and potentially by installation of rechlorination stations. In addition, systems should conduct hydraulic studies to determine the potential for sedimentation and whether additional chlorine residual sample sites need to be added or sites should be redistributed to better locate sections of the distribution system that need more attention.
ACKNOWLEDGEMENTS
The authors thank the New Jersey Department of Environmental Protection for supporting communication with the water utility and assisting with intervention activities.
DECLARATION OF INTEREST
None.







