INTRODUCTION
Leptospirosis is an infectious disease caused by a spirochete belonging to the genus Leptospira that comprises about 250 serovars, gathered in several serogroups according to antigenic community. It can infect humans and a number of mammals through contact with contaminated materials like water or wet soil [Reference Faine1, Reference Levett2]. After penetration into the blood through intact or broken skin or mucous membranes, the spirochete multiplies and reaches its target organs or tissues: kidneys, liver, lungs and cerebrospinal fluid. The associated symptoms range from dengue-like forms to the severe Weil's syndrome and fatal issues [Reference Ashord3]. Many domestic animals continue to carry Leptospira in their kidneys and shed it in their urine. Rodents, for example, are known to be powerful reservoirs.
Leptospirosis is considered as a re-emerging infectious disease, particularly in tropical and subtropical regions [Reference Levett2, Reference Meslin4]. It is endemic to Guadeloupe, an island of the French West Indies (Fig. 1). The warm and wet climatic conditions boost the spread of this infection. Indeed, a positive correlation between precipitation and incidence of leptospirosis has been observed in the region [Reference Giannini, Kushner and Cane5]. The incidence rate has been about 5–12/100 000 inhabitants since 1991 and has accounted for 8·8 deaths/1 000 000 inhabitants per year. Over a period of 3 years, the incidence grew steadily from 11·2/100 000 in 2001 to 22·9/100 000 in 2002, 33·1/100 000 in 2003 and reached a peak of 41·2/100 000 in 2004 before decreasing in 2005 to 2003 rates (data from National Reference Centre for Leptospira, Pasteur Institute, Paris, France). Interestingly, the El Niño phenomenon occurred twice during this period (principally in 2002 and at a lower level in 2004). The El Niño Southern Oscillation (ENSO) is a cyclically recurrent disruption of the oceanic/atmospheric system in the tropical Pacific. Some ENSO-related sea surface temperature anomalies influence the air–sea interactions in the North Atlantic, the Gulf of Mexico and the Caribbean Sea. In the eastern part of the Caribbean basin (Greater and Lesser Antilles) the tendency is for a drier-than-average condition during the dry season, which coincides with the mature phase of ENSO. Conversely, positive precipitation anomalies occur from the following spring onwards. The influence of the phenomenon can last up to 18 months [Reference Herrmann-Storck6].

Fig. 1. Caribbean Sea and West Indies
The aim of this study was to detect changes in the epidemiology of leptospirosis in these years of high incidence by comparison to a former descriptive analysis of patients hospitalized with symptoms of leptospirosis in the Hospital of Pointe à Pitre from 1994 to 2001 [Reference Giannini, Kushner and Cane5]. We particularly focused on the following parameters: climate, clinical manifestations, demographic characteristics, exposure to known risk factors of leptospirosis, and the involved Leptospira serogroups. Meteorological data were coincidentally analysed (Meteo-France, unpublished data). Furthermore the isolation of Leptospira strains from a large number of human cases since 2002, followed by antigenic and molecular identification allowed a more precise approach to leptospirosis in a tropical island, and highlighted the role of previously unknown Leptospira serovars in this Caribbean island.
Altogether, our data suggest that the observed epidemiological changes in leptospirosis may be linked to the El Niño phenomenon and its impact on the rodent population.
MATERIALS AND METHODS
Situation
The Guadeloupe archipelago, French West Indies, is composed of three main islands: Grande-Terre, Basse-Terre and Marie Galante. Our study concerned patients hospitalized in the Hospital of Pointe à Pitre (1100 beds), located in the middle of the largest urban community (154 000 inhabitants) of Grande-Terre (268 000 inhabitants). Another hospital (270 beds), which is not included in the study, is located at the south of Basse-Terre.
Sugarcane fields and banana plantations, cattle rearing and pig breeding are widely found throughout Guadeloupe.
Epidemiological study
From January 2003 to December 2004, physicians collected a set of data from all patients hospitalized with symptoms of leptospirosis, including: age, gender, occupation, location of residence, clinical manifestations, exposure to animals and to fresh water. At the same time, serological tests and blood culture, in media recommended for Leptospira were performed. These data were compared to those from a former study covering 1994–2001 [Reference Herrmann-Storck6].
Occupations that were considered at risk of infection by Leptospira included those leading to contacts with domestic or wild animals, fresh water or soil (e.g. agriculture, construction, dog training, fish breeding, farming and rodent control).
Rainfall data registered at the Raizet meteorological station near Pointe à Pitre were provided by Meteo-France, the French National Institute of Climatology.
Laboratory investigations
Serological tests and the identification of strains were performed at the National Reference Centre of Leptospirosis (Institute Pasteur, Paris, France). Specific antibodies were detected by the reference method of the microscopic agglutination test (MAT) [1] using a battery of live antigens including 16 Leptospira serovars. Moreover, the National Reference Centre devised an enzymatic immunological assay (EIA) with a purified antigen of the saprophytic strains Patoc to determine IgM titre [Reference Postic7]. The cut-off for a positive IgM was a titre of 1/400. A titre <1/400 was considered negative. However, given its lack of performance with Grippothyphosa and Australis serogroups, the interpretation of a negative test was never done without performing the MAT. Leptospira grew from blood samples in Ellinghausen McCullough Johnson Harris (EMJH) medium [Reference Ellinghausen and McCullough8].
We diagnosed a case of leptospirosis if the blood culture was positive or if the IgM titre was ⩾1/400 for Leptospira or if a minimal fourfold increase in MAT antibody titre was observed between acute and convalescent sera. We determined that a patient was a non-case if MAT and IgM for Leptospira were negative in acute and convalescent sera or negative in one serum at least 20 days after the onset of the symptoms.
The Leptospira genus was first categorized according to immunological grouping (serovars brought together in serogroups) and then according to DNA variation (species differentiation). Strains, which were previously classified in one serogroup, now belong to different species. Conversely, strains from different serogroups now belong to the same species. However the serogroup identification remains pertinent and commonly used. We identified the serogroup responsible for the acute case of leptospirosis as the one corresponding to the highest titre in the latest available serum. Moreover, when available, the identification of isolated Leptospira serogroups was performed by agglutination test. Pulsed-field gel electrophoresis (PFGE) after restriction with NotI enzyme was used to identify the Leptospira serovar as previously described [Reference Herrmann9]. In case of poor growth, DNA was extracted from culture medium by using the Qiagen method, and sequencing of PCR products of the 16S ribosomal genes identified the Leptospira spp. [Reference Postic10].
Statistical analysis
Epi-Info version 6.02 (Centers for Disease Control and Prevention, Atlanta, GA, USA) was used for entering data and generating univariate descriptive analysis. The χ2 test or Fisher's exact test were used for qualitative data. For comparison of quantitative variables, the Student's t test or non-parametric tests were applied, as appropriate.
The descriptive seasonal analysis was performed with Excel Microsoft version 97. Pearson's correlation was used to assess the association between rainfall and the number of cases (SPSS 15.0; SPSS Inc. Chicago, IL, USA).
Comparison of data from two periods was used for epidemiological evolution analysis (1994–2001 and 2003–2004). Potential exposure to Leptospira was known for both cases and non-cases in 2003, therefore risk factors for infection could be analysed. Those risk factors could be compared to the risk factors known in 1994–2001. Variables found to be relevant and associated with infection in 2003 were entered in a multiple stepwise logistic regression model. Adjusted odds ratios and 95% confidence intervals were calculated. Statistical significance was set at 5% and analyses were performed with SPSS 15.0 (SPSS Inc.).
RESULTS
Sixty-two cases were confirmed in 2003 among 222 suspected patients, whereas 103 cases were confirmed in 2004 among 266 suspected patients (respectively 44·2% and 85% of all cases in Guadeloupe notified by the National Reference Centre).
Seasonal variations of leptospirosis incidence
The monthly distribution of incidence was constant from 1996 to 2001, with the lowest incidence from March to May, an increase from July to September and a peak of incidence recorded from September to December (Fig. 2). The Figure displays the succession of the events: the cycle of transmission begins about 1 month after the onset of sustained rainfall (except after Hurricane Lenny in November 1999). After the El Niño phenomenon in 2002 drought occurred from November 2002 to May 2003, followed by a very wet and hot season. During the weak 2004 El Niño episode, humidity remained unusually constant and high with no dry season, while in 2005, only one month of dry conditions occurred (in March). Concomitantly, leptospirosis incidence did not fall to its low endemic minimum baseline, and reached maximum-level records during the high endemic season.
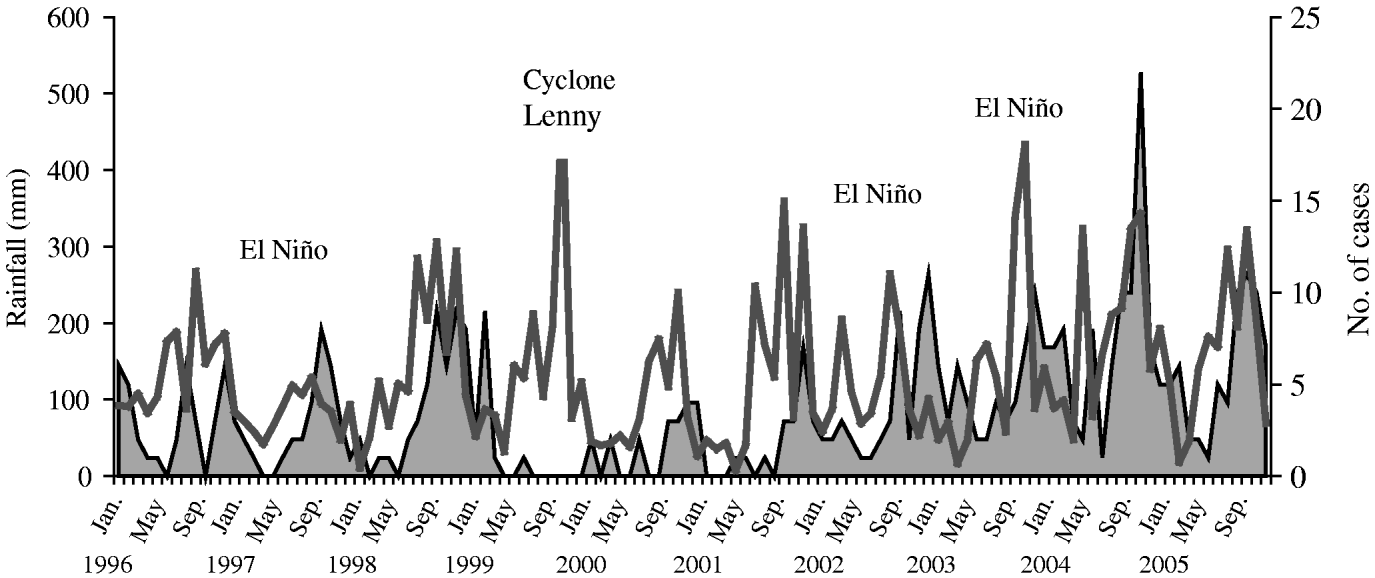
Fig. 2. Monthly rainfall (![]() ) and leptospirosis cases (
) and leptospirosis cases (![]() ) for 1996–2005.
) for 1996–2005.
Population characteristics
In 2003–2004, the mean age of the tested population was unaltered compared to 1994–2001 (48·2±6 years vs. 43·3±1·2 years in 1994–2001). However, the mean age of cases in 2003–2004 was significantly higher than in 1994–2001 (51·27±4·7 years vs. 43±2·8 years, P<0·05). When the number of 15- to 30-year olds tested for leptospirosis or confirmed cases fell in 2003–2004, middle age remained the prominent class among tested and confirmed infected patients. Indeed, we noticed an increased incidence in older populations corresponding to people aged >50 years (Fig. 3).
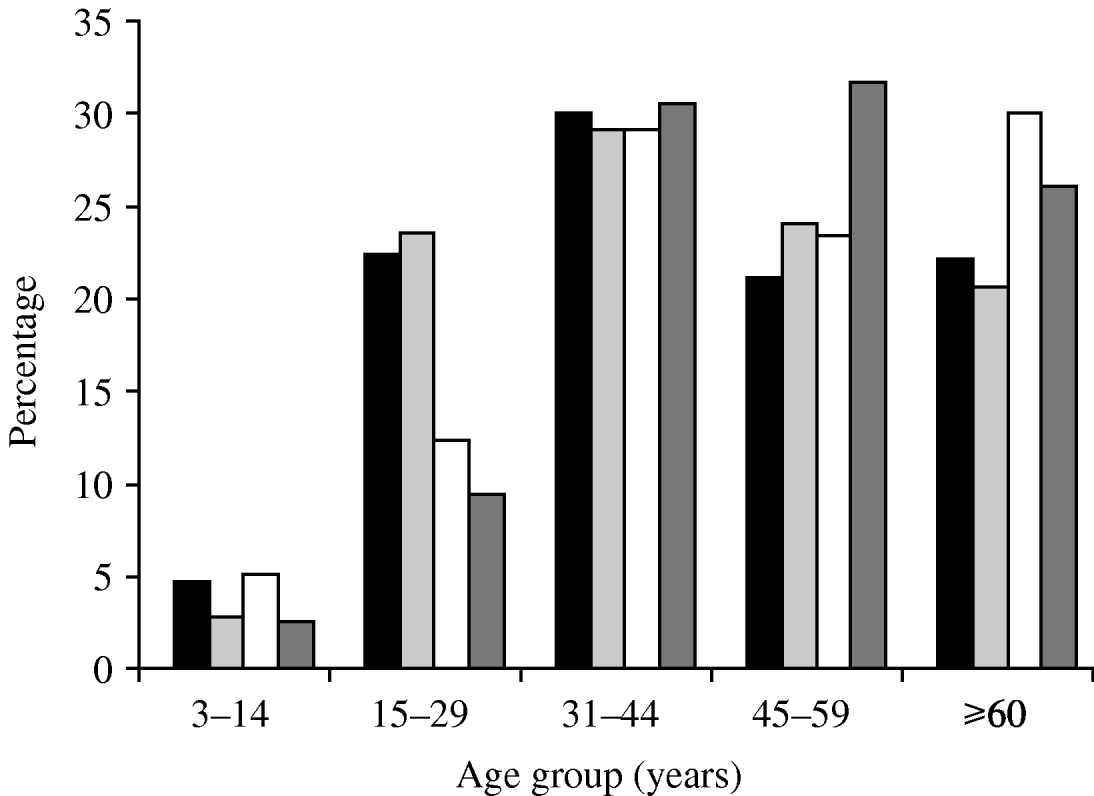
Fig. 3. Percentage of patients tested for leptospirosis and percentage of confirmed cases of leptospirosis by age group during 1994–2001 (■, tested; ![]() , cases) and 2003–2004 (□, tested;
, cases) and 2003–2004 (□, tested; ![]() , cases).
, cases).
The predominance of males among cases was reinforced, although not significantly (male/female sex ratio: 6·36 in 2003–2004 vs. 4·3 in 1994–2001).
Clinical and biological features
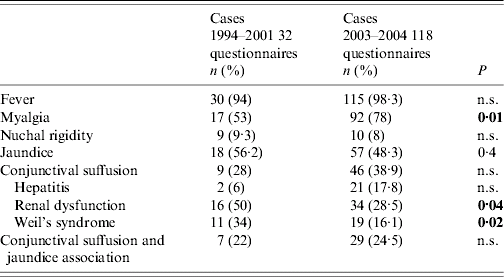
Clinical and biological data from leptospirosis cases collected in 2003 and 2004 were available for 118 cases (Table 1). They revealed that severe forms of the disease were less frequent in 2003–2004 than previously. In particular, the incidence of renal dysfunction (50% in 1994–2001 vs. 28·5% in 2003–2004, P=0·04) and Weil's syndrome (34% in 1994–2001 vs. 16·1% in 2003–2004, P=0·02) decreased significantly during the last period.
Table 1. Comparison of clinical and biological data of leptospirosis between 1994 and 2001 (212 cases) and 2003 and 2004 (165 cases) in the Teaching Hospital of Pointe à Pitre, Guadeloupe, French West Indies
Exposure to risk factors
As observed in the 1994–2001 survey, occupations having contact with fresh water, soil, rodents or cattle had a high risk factor for leptospirosis in 2003 (55·8% of cases) (Table 2). Farmers and masons were the most frequent occupations in this group (43·5% and 25·6% respectively). Fresh water contacts during leisure became more frequent among tested people in Guadeloupe than previously, but without association with leptospirosis in 2003 (31% in cases vs. 19·2% in non-cases, P=0·14). It remained almost constant in 2004 (41·5% in cases). Univariate analysis confirmed that cases more frequently reported contacts with rodents (52%), pigs (37·2%) and cattle (37·2%) than did non-cases. These figures were similar in 2004 (47% contact with rodents, 33·8% contact with pigs). After stepwise logistic regression including six variables, contact with pigs (P<0·001) and contact with rodents (P=0·02) were found to be independent risk factors for leptospirosis (Table 3). This represents a noticeable change since contact with pigs and cattle, but not rodents, were risk factors for leptospirosis in the previous study in Guadeloupe [Reference Giannini, Kushner and Cane5].
Table 2. Occupational exposure risks to leptospirosis transmission in 2003 compared to 1994–2001 in the Teaching Hospital of Pointe à Pitre, Guadeloupe, French West Indies
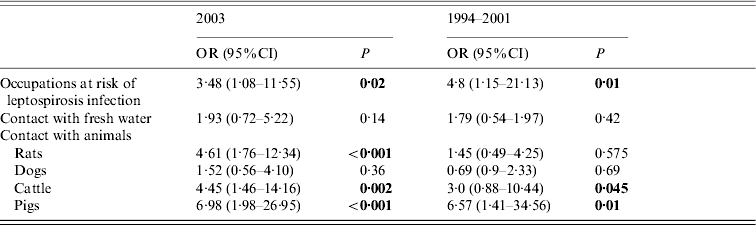
OR, Odds ratio; CI, confidence interval.
Table 3. Results of multivariate stepwise logistic regression analysis of risk factors for leptospirosis in 2003
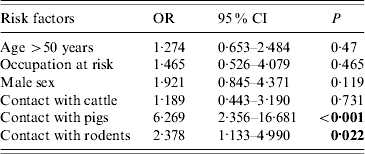
OR, Odds ratio; CI, confidence interval.
Leptospira serogroups, serovars and species
The presumptive serogroups responsible for acute leptospirosis are shown in Table 4. The serogroup was identified in 117/165 cases in 2003–2004 (71%). Culture allowed confirmation of the serogroup in 52 cases and identification of the serovar in 46 cases. Results emphasized a dramatic increase of the Ballum serogroup, which became the second most common (32%) after the Icterohaemorrhagiae serogroup (56%) while the Australis serogroup remained roughly constant at around 5–10% (Fig. 4). PFGE was performed on DNA extracted from 54 isolated strains. It asserted the predominance of the Icterohaemorrhagiae and Arborea serovars. It also allowed the discovery of the role of the Mini serogroup. The use of 16S rDNA sequencing for seven strains unveiled one Leptospira santarosai, one Leptospira noguchii and five Leptospira kirshneri strains. Serogroups Cynopteri, Tarassovi, Sejroe, Canicola and Hebdomadis were rare and only serologically suspected.
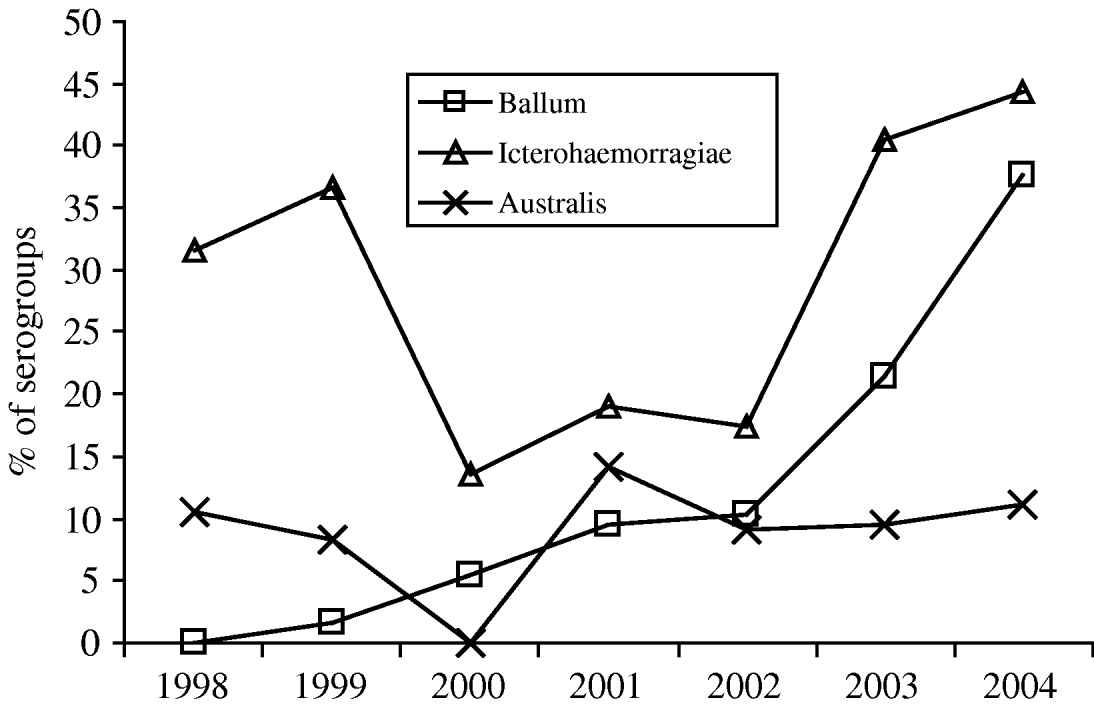
Fig. 4. Evolution of the proportion of the main serogroups responsible for human cases of leptospirosis (1998–2004) in Guadeloupe, French West Indies.
Table 4. Leptospira sensu lato serogroup and serovar distribution among 117 cases of patients in Hospital of Pointe à Pitre, Guadeloupe, French West Indies, in 2003–2004
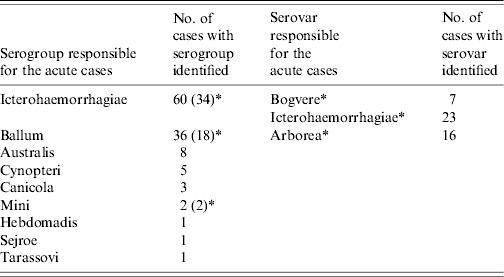
* Identified with culture.
Leptospira serogroups and symptoms
There were few data to assess the association of serogroups and symptoms but jaundice was more commonly associated with the Icterohaemorrhagiae serogroup than the Ballum serogroup (53·2% vs. 28%, P=0·03), as was renal dysfunction, although not significantly (34·2% vs. 24%, p=0·3).
Leptospira serogroups and exposure to risk factors
The study of the links between exposure and the serogroup responsible for the infection could be performed for 15 Ballum serogroup infected cases and 30 Icterohaemorrhagiae serogroup infected cases. Ballum serogroup infections were linked to animal contacts more than Icterohaemorrhagiae serogroup infections. Contact with rodents was recorded in 53% of cases of Ballum infection vs. 38% of cases of Icterrohaemorrhagiae infection, with pigs in 40% of cases vs. 27%, and with cattle in 44·4% of cases vs. 27·6%, although not significant differences. In contrast, the Icterohaemorragiae serogroup appeared to be connected to contact with fresh water more often than the Ballum serogroup (66·6% vs. 33·3% respectively) although not significantly. The Ballum serogroup was also more frequent among the older population (48·1% of people aged ⩾60 years vs. 26·2% of people aged <60 years, P=0·03) who are also in contact with rodents more often (64·3% of people aged ⩾60 years vs. 45·2% of people aged <60 years, P=0·05).
DISCUSSION
The number of leptospirosis cases was abnormally high in 2003 in comparison with former data available since 1994 and rose steadily in 2004 even during the months of usually lower incidence. In 2005, the figure fell but remained close to the level of 2003.
In 2004 the National Reference Centre of leptospirosis changed the threshold of MAT positivity from 1/100 antibody titre to 1/400 to improve the specificity of the test in the endemic region of Guadeloupe. This explains why the number of cases registered by the National Reference Centre (including every case in Guadeloupe) fitted better with the number of cases registered in the Hospital of Pointe à Pitre in 2004 than in 2003.
Interestingly, during this period of dramatic increase in leptospirosis incidence, two consecutive El Niño phenomenon occurred (2002–2003 and 2004–2005). Given their potential positive impact on the rainfall pattern in the Caribbean region and the disappearance of a real drought period over 3 years, it is possible that those unusual climatic conditions boosted Leptospira transmission. The prevalence of leptospirosis still doubled in 1999 in Guadeloupe after a 1997–1998 El Niño phenomenon (13 cases/100 000 inhabitants vs. an average of 4·5 cases/100 000 inhabitants during the six previous years) (National Reference Centre of Leptospira annual reports, Pasteur Institute, France). However, the meteorological models to explain the worldwide effects of ENSO are still matter of study and further observations need to be performed before firm conclusions can be drawn about the linkages between El Niño occurrence and leptospirosis incidence in the Caribbean islands. Numerous studies have shown that the El Niño phenomenon was associated with an increased risk of transmission of vector-borne diseases such as malaria [Reference Poveda and Rojas11], dengue [Reference Hales, Weinstein and Woodward12] and yellow fever [Reference Diaz and McCabe13]. It is supposed that the El Niño phenomenon optimizes humidity and temperature for the multiplication of mosquito vectors [Reference Hales14, Reference Haines15]. Interestingly, West Nile virus emerged in Guadeloupe among horses during 2002 [Reference Quirin16]. Similarly, the El Niño phenomenon could have influenced rodent-borne diseases in the United States where an increased incidence of hantavirus pulmonary syndrome was associated with an increase in the rodent population [Reference Engelthaler17, Reference Berlioz-Arthaud18]. Such a phenomenon could have occurred in Guadeloupe, increasing the reservoir of Leptospira. In contrast heavy rains during cyclones did not induce a peak of leptospirosis incidence perhaps due to destruction of the environmental reservoir and the vectors' habitation [Reference Giannini, Kushner and Cane5]. The consequences of such disasters in this comparatively affluent island cannot be compared to the devastating aftermath of flooding in poor countries, where habitations and the urban environment can be devastated for months. In such conditions, an increase in the rodent population and the rise of Leptospira transmission will inevitably occur.
An increased ability of physicians to diagnose leptospirosis could not explain the growth of incidence in 2003–2004, as the ratio of cases towards the total number of tested patients was the same during 1994–2001. However, improvements in the clinical and biological follow-up of patients probably led to a better access to diagnosis and a more precise identification of Leptospira strains responsible for the disease. The infection shift to the older population group has been observed in other industrialized countries, e.g. Germany, because of the predominance of residential exposure risks [Reference Berlioz-Arthaud18]. In Guadeloupe, cattle breeding is a common occupation for males aged >50 years and retired people. This trend could thus reflect the reinforcement of this risk factor linked to the climatic conditions and therefore to rodent proliferation.
The drop in renal dysfunction and severe disease forms like Weil's syndrome is surprising considering the ageing infected population. However, several explanations can be put forward. The Ballum serogroup appears rarely associated with severe renal dysfunction in patients in 2003–2004 compared to the Icterohaemorrhagiae serogroup. This finding was previously observed in Guadeloupe [Reference Jansen19]. Moreover, because of the fear of complications, older people suspected of infection are more readily hospitalized by their general practitioner, leading to early treatment, which avoids severe complications.
The predominance of the Icterohaemorrhagiae serogroup in Guadeloupe was already known. The Australis serogroup, which is the main serogroup, found in pigs in 2002 was also abundant (A. Scoizec, personal comunication). This serogroup accounted for 28% of total leptospirosis in a 2003–2005 multicentre survey in the Western Pacific [Reference Strobel20]. In this region pig farming might be a high-risk activity as in Guadeloupe. The Ballum serogroup occurred in 16% of human leptospirosis cases that have been reported in Guadeloupe since 1994 (National Reference Centre of Leptospira annual reports, Pasteur Institute, France). This serogroup has less implication in the other Caribbean islands [Reference Everard and Everard21–Reference Lhomme23]. Its sharp growth since 2002 could be in accordance with the increasing role of rodents in disease transmission. Accordingly, the Arborea serovar within the Ballum serogroup was predominantly found among a large population of mice in a study of Leptospira wild carriers conducted in Guadeloupe in 2001 (V. Michelle, personal communication). It was also found in 10% of Rattus spp. that were captured in the neighbourhood of nine human cases in 2003 (data not shown). These data on Arborea confirm those reported from Barbados [Reference Matthias and Levett24]. Interestingly, our study highlights a correlation between Ballum serogroup infections and animal contacts as well as a significantly higher exposure to rodents and the predominance of the Ballum serogroup among patients aged ⩾60 years. The other hypothesis for this correlation between Ballum and older cases could be exposure to mice in the home. The characterization of numerous Leptospira strains in a short period was very useful in assessing the role of species other than L. interrogans in human disease. Thus, we established the presence in Guadeloupe of L. noguchii, L. kirshneri (serovar Bogvere, first isolated in Jamaica) and L. santarosai previously found mostly in South America. Similarly, the serogroup Mini, so far unknown in Guadeloupe, was identified as an agent of human leptospirosis for the first time in this region. Otherwise, the spectrum of Leptospira strains responsible for human infection seems to be narrower than previously thought. Isolated Leptospira strains that were identified by PFGE belonged to the three main serovars Icterohaemorrhagiae, Bogvere and Arborea. The role of serogroups Cynopteri, Tarassovi, Panama, Grippotyphosa and Automnalis was minor. The island model as a small area with numerous rodents and domestic hosts where restricted variety of strains are circulating should match with the epidemiology of leptospirosis in Guadeloupe island and must be further studied and evaluated.
CONCLUSION
Recent changes in the epidemiology of leptospirosis in the tropical island of Guadeloupe seem to be highly related to climatic conditions, with high potential for a rapid burst of transmission and a possible impact on serogroups responsible for infection, on targeted human population, and perhaps on the clinical feature of the disease. Establishment of a model of leptospirosis epidemiology, based on climatic and perhaps rodent population monitoring could help the management of this disease in the island [Reference Mills and Childs25]. Moreover, strain surveillance among infected humans and animals must be pursued to discover the spectrum of the species circulating. This will allow adaptation of serological diagnostic resources and help in understanding the dynamics and possible changes in Leptospira transmission.
ACKNOWLEDGEMENTS
We thank Meteo-France for rainfall data, especially Mr Gérald Martin for his personal commitment in helping us to study the links between climate and leptospirosis in Guadeloupe. We thank also the physicians in the hospital of Pointe à Pitre whose efficient work of clinical diagnosis and data collection has made it possible to perform this analysis, and Dr Jacqueline Deloumeaux for her effective help in multivariate analysis and Pearson correlation.
DECLARATION OF INTEREST
None.










