Introduction
The battle against Campylobacter a few years ago seemed quite challenging, although it showed promising advances. The success of Salmonella eradication programmes and the rapidly growing knowledge on the characteristics of Campylobacter promised effective control tools in the near future. Although Salmonella eradication programmes have been effective in most of the countries, this is not true for Campylobacter. Most European Union (EU) Member States (MSs) have not developed national control programmes for this pathogen and the prevalence of Campylobacter infection continued to increase [1, Reference Kaakoush2].
The present number of confirmed human campylobacteriosis cases is almost two and a half times higher than the number of salmonellosis cases in the EU. The overall disease burden of campylobacteriosis in the EU is approximately 0.35 million disability-adjusted life years (DALYs) that can vary between <10 DALY/100 000 to >100 DALY/100 000 people in the MSs [Reference Mangen3, 4], and it is considered to be a priority issue by the European Commission (EC). The introduction of a microbiological criterion was discussed as a solution to this issue several times in the past. However, the epidemiological status of MSs is diverse and only limited information is available on the epidemiology of Campylobacter spp. or on effective control measures in these states [Reference Mangen5]. Therefore, common mandatory control programmes for farms or a food safety criterion for products were considered to be not applicable. The EC is still facilitating all MSs to start their own control programme depending on the epidemiological status of each country, by establishing a process hygiene criterion for Campylobacter in broiler carcasses as an amendment to the Regulation (EC) No. 2073/2005 [6].
According to the annual report of the European Food Safety Authority and the European Centre for Disease Prevention and Control (EFSA and ECDC) on zoonosis monitoring activities carried out in 2015 [1], campylobacteriosis is the most common zoonosis in the EU. The annual number of confirmed human cases is above 220 000 and the notification rate per 100 000 population was 65.5. Regarding country-related results, the highest notification rate was associated with the Czech Republic, Slovakia, Sweden and the UK (198.9, 128.2, 94.2 and 92.2, respectively).
The clinical symptoms of human campylobacteriosis are typically limited to a mild gastroenteritis (GE) episode; however, severe and even fatal GE cases may also occur. Long-term complications are rare but can also be severe/fatal and may include Guillain–Barré syndrome (GBS) and reactive arthritis [Reference Havelaar7–Reference Kemmeren9]. Inflammatory bowel diseases and post-infectious irritable bowel syndrome have also been raised as potential long-term complications of human campylobacteriosis [Reference Mangen8–Reference Mangen11], but with less evident causal associations [12].
Reported data regarding the proportion of campylobacter-positive animals did not comprehensively cover all of the MSs. Only six countries (five MSs and one non-MS) reported prevalence data on pigs, and six MSs reported data on cattle (with prevalence ranging between 0% and 73.1% and between 0% and 64.2%, respectively). Only five countries reported data on broilers that were related to slaughter batches, flocks or animals. Campylobacter was found in 19.3% of the units tested. It is important to emphasise that country-specific data are not directly comparable because of the differences between the monitoring systems of MSs.
Nevertheless, evidence suggests that campylobacteriosis is one of the most important human foodborne infections, attributable to broiler chicken consumption in about 20–30% of cases [13]. Although the effect of interventions varies between MSs, a three log reduction in the number of Campylobacter in the intestines at slaughter could reduce the public health risk by 90% [4, Reference Nauta14].
A couple of quantitative risk assessments have been previously published on Campylobacter within-flock and between-flock transmission on farms; on Campylobacter concentration changes in and on broiler carcasses and meat during broiler processing; and on transfer and survival of Campylobacter in households prior to food consumption [Reference Nauta15, Reference Chapman16]. However, models of the human clinical and economic burden due to foodborne campylobacteriosis are sparse in the literature. Following systematic research on the prevalence of Campylobacter in the broiler chicken supply chain in the EU and the identification and efficacy characterisation of alternative control measures, EFSA Scientific Opinions initiated by the EC were formulated in 2010 and 2011 [4, 13]. Before that, the Campylobacter risk management and assessment project advised the Dutch government on the effectiveness and efficiency of interventions aimed at reducing campylobacteriosis cases and developed a cost-utility analysis in the Netherlands [Reference Mangen5, Reference Mangen, de Wit and Havelaar17–Reference Katsma19]. As a next step, DG SANCO of the EC initiated a project to analyse the socio-economic costs and benefits of applying certain control measures in any of the EU-27 MSs for the reduction of Campylobacter in broiler meat in the different stages of broiler production and food supply chain [20, 21]. According to this European-level cost-utility analysis, implementation of currently available food safety technologies with good consumer acceptance (Fig. 1) in the food chain of indoor broiler chickens would result in 67 300 annual avoided DALYs and €353 million annual cost savings in the EU-27. Comprehensive application of the EC adopted economic model in food safety policy-making is expected to guide decisions on Campylobacter programmes in the broiler sector at the EU and/or at national levels. In this context, the validity of modelling assumptions is essential; therefore, we have conducted a critical review of model input parameters on health burden and economic burden of human campylobacteriosis. These parameters were selected to be reassessed because there were neither built-in selectable options nor country-specific adaptations for these assumptions in the model, unlike for other important input parameters such as the effectiveness and costs of intervention options, or broiler industry structure by country.
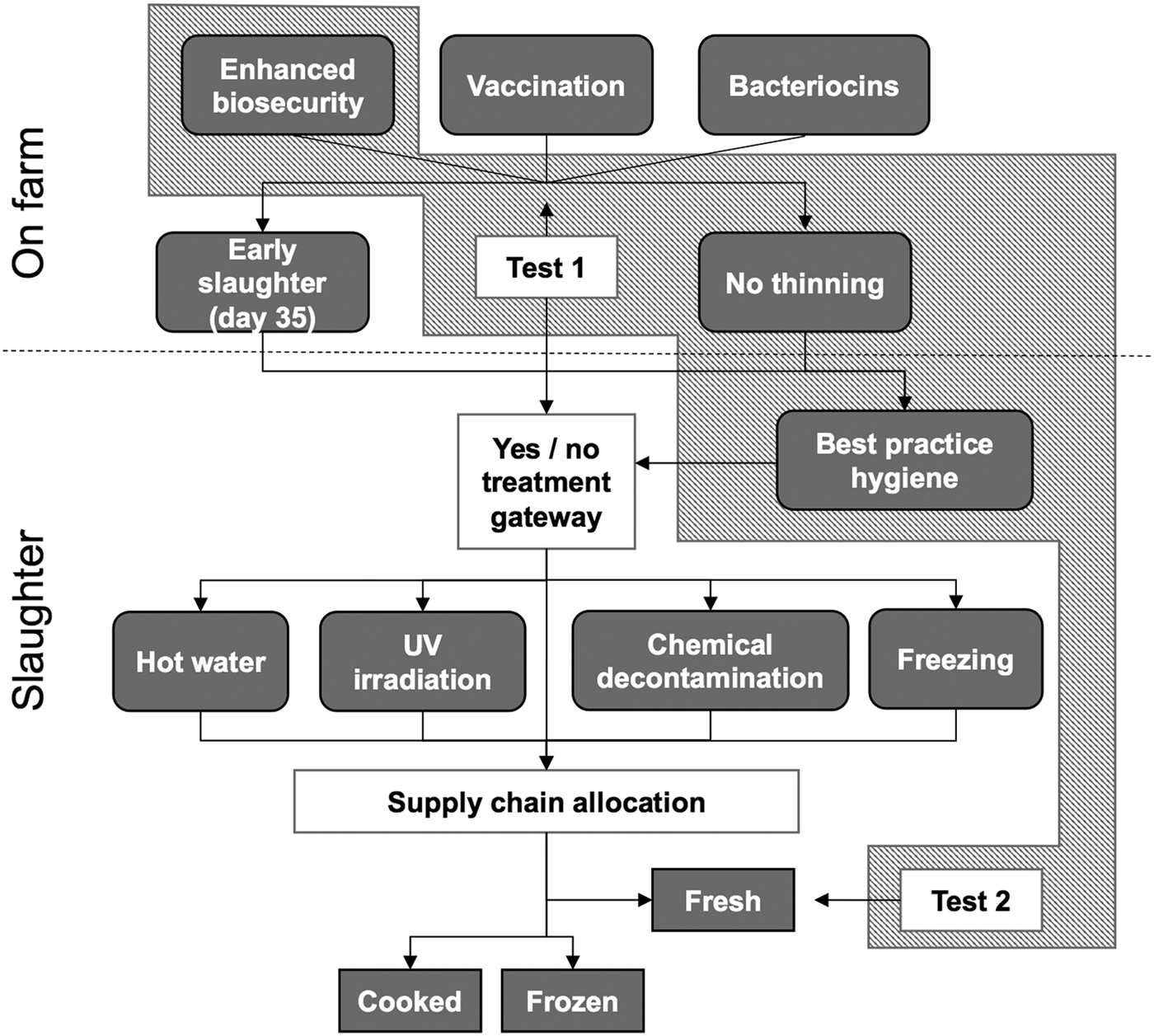
Fig. 1. Potential food safety interventions in the food chain of indoor broiler chickens. Currently available food safety technologies with good consumer acceptance are highlighted by the polygon with diagonal pattern. Adapted from: [20].
The health burden in the published model was expressed in DALYs, similarly to most analyses in food safety risk management. Interestingly, the recent systematic re-evaluation of foodborne disease burden in the USA applied quality-adjusted life years (QALYs) instead of DALYs as health burden metrics [Reference Scharff22–Reference Batz, Hoffmann and Morris24]. Although both DALY and QALY are population health metrics describing morbidity and mortality simultaneously in a single number, they were developed with different intentions and are not interchangeable. DALY was developed to describe health at the population level, without the aim of responsiveness to slight health changes at the individual level. In contrast, the primary aim of developing the QALY methodology was to support the evaluation of medical interventions [Reference Gold, Stevenson and Fryback25]. Since QALY became dominant, almost exclusively used health denominator in Health Technology Assessment, Pitter et al., [Reference Pitter26] suggest that cost-utility analyses in Food Safety Risk Analysis should also adopt QALY for the standard quantification of the health impacts of food safety policies.
The specific objectives of the present study were (1) to conduct an independent review of two key input parameters (health burden and cost of illness per human case) of the EU-27 level campylobacteriosis model [20, 21]; (2) to develop an EU health burden per case estimate nominated in QALY units; (3) using the above adapted model input assumptions, to re-evaluate the cost-effectiveness of the EU-wide implementation of a Campylobacter control strategy consisting of all currently available food safety technologies with good consumer acceptance; and (4) to conduct cost-utility analyses of bacteriocin treatment and vaccination, two alternative preventive strategies under development and with anticipated good consumer acceptance. Sensitivity analyses with lower intervention effectiveness of the selected strategy are also provided, together with the discussion of putative implementation barriers and opportunities.
Methods
The EU-27 level cost-utility model on alternative Campylobacter control measures for the indoor broiler chicken sector was downloaded from the EC webpage [20, 21]. This model runs in a Microsoft Excel platform and allows selections among several interventions (Fig. 1) to be virtually implemented in any combination of EU-27 MSs. Reflecting the uncertainty in the effectiveness of the potential interventions, the user may select from three efficacy assumptions for each of the interventions based on the lowest reported efficacy, a midpoint estimate and the highest reported efficacy data for the particular intervention option. Costs of interventions are also adjustable in the model, to allow custom sensitivity analyses. The model is adjustable to changes in broiler industry structure (e.g. number of birds, percentage of products frozen, wages, etc.) with country-specific base case data available in the model input data sheets. However, the health and economic burden of a human campylobacteriosis case is kept constant in the model across all countries, without built-in sensitivity analysis options.
Interestingly, most of the included control measures were reported to be of high consumer impact (i.e. poor consumer acceptance) or being under development [20]. Consumer education/communication efforts might change the consumer attitude towards currently poorly accepted control measures (e.g. hot water treatment) in the future, which would be advantageous for consumer safety given the high risk of cross-contamination at the slaughter process. However, our analysis focused on the combination of all currently available interventions having good consumer acceptance at present, i.e. ‘enhanced biosecurity’ and ‘no thinning’ at farms and ‘best practice hygiene’ in slaughterhouses, together with two main testing points test 1 and test 2 as proposed in the original model (Fig. 1). In this scheme, the first test point (T1) would be undertaken on farms shortly before sending the flock to slaughter, to measure the success of on-farm control strategies and to inform the processing plants about the Campylobacter status of the flock. The proposed approach is to let farm staff submit a pooled sample of 30 fresh faecal droppings from each house for laboratory analysis. The second test point for Campylobacter (T2) would be undertaken at the processing plant, focusing on whole carcasses and portions that are marketed as fresh rather than frozen. Sampling would be undertaken immediately after processing by the QC personnel at the plant [20]. Enhanced biosecurity at farms would consist of improved training and different set of boots for each house with changing facilities. The current uptake of these measures was set to 10% in most MSs, except for Denmark, Estonia, Finland, the Netherland and Sweden where 50% baseline uptake was assumed based on low campylobacteriosis incidence [20]. The maximum uptake was assumed to be 95% in all MSs, and the selectable effectiveness assumptions of enhanced biosecurity are 40%/55%/70% reduction in broiler colonisation, based on the prior EFSA opinion of 38–71% that was built on UK data only [20, Reference Gibbens27]. For this model, thinning was defined as the practice of removal of 25% of a flock at day 35, followed by full depopulation at day 42. The baseline uptake of ‘no thinning’ was set to 25% in all MSs, except for Sweden (95%) where thinning was banned [20]. Maximum uptake was set to 95% in all MSs except for France (75%) where 25% of broilers are grown outdoor [20]. The selectable effectiveness assumptions on no thinning were 10%/18%/25% reduction in broiler colonisation [4, 20]. Best practice hygiene at processing plants is expected to reduce the contamination of carcasses with Campylobacter by 20%/25%/30%, based on a single study conducted in nine Belgian slaughterhouses [20, Reference Habib28]. For this model, a 10% baseline uptake and a 100% maximal uptake of best practice hygiene was assumed in all MSs [20].
As an alternative strategy, the expected value of bacteriocin treatment or vaccination of the animals was also investigated, including the same testing scheme as detailed above. Bacteriocin treatment and vaccination presumably will also have a good consumer acceptance but are still under development. Accordingly, their baseline uptake was set to 0%, with a maximal uptake of 95% in all MSs except for France (75%). Selectable options for the efficacy of both vaccination and bacteriocin treatment are 50%/70%/90% reduction in broiler colonisation, based on the available evidence [Reference de Zoete, van Putten and Wagenaar29].
For all analyses, the published model structure and base case input parameters were left unchanged, except for the input variables on health and economic burden as described in the corresponding Methods subsections; and lower intervention effectiveness assumptions in the sensitivity analysis (see at Results).
A QALY-based, European disease burden estimate for human campylobacteriosis
The EU-27 proportions of mild, moderate, severe and fatal GE events were assumed to be 76.27%, 22.72%, 0.97% and 0.0424%, respectively, based on the data from the Netherlands [Reference Kemmeren9]. The corresponding EQ-5D scores were retrieved from the literature [Reference Havelaar7] and were translated into disutility weights by a utility mapping function established in the combined analysis of 11 population surveys in six EU countries [Reference Greiner30]. Outcome probabilities were multiplied by the corresponding disease durations (data from the Netherlands [Reference Kemmeren9]) and disutility weights, to calculate the attributed health burden (in total, 11.58 QALY loss in 1000 human GE cases; Table 1). Note that the health burden in fatal GE cases occur far beyond 1 year. In health economic analyses, costs and benefits occurring in the future are generally considered less valuable than those occurring in the present, and the gradual devaluation of future costs and benefits is typically achieved by applying an annual discount rate. An important reason for discounting future costs/gains and benefits/harms in an economic analysis is time preference, i.e. the general desire to enjoy benefits in the present while postponing any negative effect [Reference Torgerson and Raftery31]. The best practice for discounting future health benefits/risks is to apply the same discount rate as for future monetary costs/savings [Reference Smith and Gravelle32–Reference Bos, Postma and Annemans34]. The published Campylobacter control model applied an annual 4% discount rate for capital, but did not discount the health benefits/losses [20, 21]. In our analyses, we applied the same 4% discount rate both for capital and health impacts, resulting in a discounted health burden due to Campylobacter GE of 9.96 QALY loss in 1000 cases.
Table 1. Calculation of QALY loss estimate for human campylobacteriosis in the EU
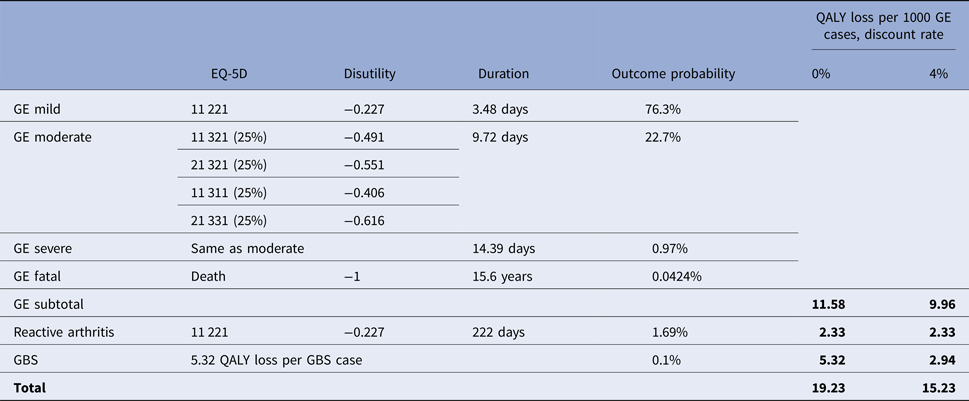
GBS, Guillain–Barré syndrome; GE, gastroenteritis. Patients with mild, moderate and severe GE cases had no medical visit, had a general practitioner visit or were hospitalised, respectively. For data sources, see the main text.
Reactive arthritis was assumed to occur in 1.69% of GE cases, with a mean disease duration of 222 days [Reference Kemmeren9] and an EQ-5D score similar to the mildest stage of rheumatoid arthritis patients [Reference Hurst35]; the latter score was translated to a disutility weight of −0.227[Reference Greiner30]. Accordingly, health burden due to campylobacter-related reactive arthritis was 2.33 QALY loss per 1000 GE cases. Discounting has no effect on this figure, since the reactive arthritis symptoms do not persist beyond 1 year.
The assessment of health burden due to GBS is complex as a result of the diversity of the underlying patient paths (need for hospitalisation, intensive therapy and/or ventilation; variability in disease duration and time to return to work) [Reference Batz, Hoffmann and Morris24]. Previous estimates of DALY burden of a GBS case in the Netherlands were in the 4.83–6.26 DALY range [Reference Havelaar7–Reference Kemmeren9], showing good numeric agreement with a recent, QALY-based GBS burden estimate in the USA (5.32 QALY loss per GBS case) [Reference Batz, Hoffmann and Morris24]. In our analyses, the European health burden of campylobacter-related GBS was estimated as 5.32 QALY loss per one GBS case [Reference Batz, Hoffmann and Morris24], multiplied by the 0.1% EU incidence of GBS after Campylobacter infections [Reference Kemmeren9], resulting in 5.32 QALY loss due to GBS in 1000 European Campylobacter GE cases (undiscounted). Note that GBS health burden remains significant beyond 1 year due to fatal cases and patients with persistent symptoms. When an annual 4% discount rate was applied, discounting was reported to result in a 43–45% overall decrease in the DALY burden of campylobacter-related GBS in the Netherlands [Reference Mangen8, Reference Kemmeren9]. Adopting the 45% overall decrease in GBS health burden due to a 4% discount rate, we estimated a discounted health burden of 2.94 QALY loss due to GBS in 1000 human Campylobacter GE cases.
Recent publications on the health burden of Campylobacter GE included post-infectious irritable bowel syndrome, accounting for 18–18.5 DALYs in 1000 Campylobacter GE cases [Reference Haagsma10, Reference Mangen11]. Thus, the inclusion of this long-term sequela nearly doubles the estimated overall health burden of human campylobacteriosis. However, according to a World Health Organization (WHO) report on expert consultations in 2012, the causative association between campylobacteriosis and functional gastrointestinal disorders/inflammatory bowel diseases has not been convincingly established yet [12]. In line with a recent update of the Campylobacter disease outcome tree in the USA [Reference Hoffmann, Batz and Morris23, Reference Batz, Hoffmann and Morris24], we decided to neglect the health burden of putative long-term gastrointestinal sequelae of Campylobacter GE in our analyses, to avoid the overestimation of health burden attributable to human campylobacteriosis.
Accordingly, the overall health burden due to campylobacteriosis was estimated to be 19.23 or 15.23 QALYs per 1000 human Campylobacter GE cases in the EU (with 0% or 4% annual discount rates, respectively). For comparison, the published model assumed a health burden of 38.9 DALYs per 1000 human Campylobacter GE cases, and did not discount the health burden estimate, while a 4% discount rate was applied for capital [20, 21].
Country-specific cost of illness estimates for human campylobacteriosis
The published model assumed 9 million annual human Campylobacter GE cases in the EU-27 with an overall cost of illness of €2400 million and with an underlying mean cost per case estimate of €267 [20]. These assumptions were based on an EFSA Scientific Opinion in 2010 [13] which referred to data from the Netherlands and Belgium, with mean cost of illness per case estimates of €261 and €495, respectively [Reference Mangen8, Reference Gellynck36]. In the Dutch study, GE, GBS, reactive arthritis and inflammatory bowel diseases accounted for 73.3%, 16.5%, <0.5% and 9.2% of the total cost of illness, respectively, whereas irritable bowel disease was not investigated. Discounting with an annual 4% discount rate had marginal effect on cost of illness estimates since most costs occurred in the first year of infection [Reference Mangen8]. Accordingly, the adjusted mean cost of illness estimate in the Netherlands (subtracting 9.2% inflammatory bowel disease-related costs) is €245 per Campylobacter GE case, with €200 and €45 mean costs related to GE and GBS, respectively. In the Dutch study, direct healthcare costs represented 10% and 70% of GE and GBS costs, respectively, while indirect costs (almost exclusively productivity loss) were responsible for the remaining 90% and 30% of these costs. Direct non-healthcare costs (including travel and informal care were found to be of minor importance (<0.5%)) [Reference Mangen8]. Therefore, the adjusted mean cost of illness estimate in the Netherlands consists of €51.5 direct healthcare costs (21%) and €193.5 cost of productivity loss (79%).
Application of these numbers to other EU-27 MSs would not take into account important country-specific differences in healthcare costs of treating GE and the included sequelae, as well as the differences in the price of productivity. On the other hand, the conduct of separate campylobacteriosis cost of illness studies in all EU-27 MSs would require large research efforts with substantial demands for scientific and monetary resources. Consequently, our analysis is based on the available data from the Netherlands with the introduction of two proxies as readily available, macro-level correction factors: total healthcare expenditure per capita at the average exchange rate [37] and gross average monthly wages at nominal exchange rates [38] to reflect country-specific differences in healthcare costs and in the price of productivity, respectively. Missing the United Nations Economic Commission for Europe (UNECE) data on monthly average wages in Cyprus and in Malta were replaced by data from Greece and Italy, respectively. The corrected country-specific mean cost of productivity loss estimates ranged from €22.3/case (Bulgaria) to €254.8/case (Luxemburg), while the mean direct healthcare cost estimates ranged from €4.2 (Romania) to €66.9 (Luxemburg) (Table 2). The mean cost of illness estimate from the Netherlands was found to be more or less representative for most Western and Northern European countries, but its extrapolation to the Southern, Central and Eastern European countries would clearly overestimate the cost of illness burden in these regions (Table 2). In this context, it is important to recognise that about 75% of the European human Campylobacter GE cases occur in the latter regions [20, 21].
Table 2. Cost of illness estimates for a human campylobacteriosis case, and fraction of annual EU-27 human campylobacteriosis cases by Member States

For cost calculation methods, please see the Methods section. Source for the incidence data: [21]. Countries with >10% contribution to the overall EU-27 incidence are highlighted in dark grey; countries with cost of illness data that was extrapolated to all Member States in the original model are highlighted in light grey.
Cost-utility analyses
In the adapted cost-utility analyses, the expected change in the number of human GE cases was determined by the original model [20, 21]. Patient numbers were multiplied by the discounted QALY loss per case and country-specific cost per case constants determined in ‘A QALY-based, European disease burden estimate for human campylobacteriosis’ and ‘Country-specific cost of illness estimates for human campylobacteriosis’ sections, to estimate the Campylobacter-related total health burden and the overall cost of illness in each MSs. Effectiveness and costs of control measures were left unchanged according to the base case scenario of the published model. All investigated strategies were compared with the current (baseline) situation. Incremental cost-effectiveness thresholds (ICER) were calculated as Δ cost/Δ QALY impacts of the investigated strategies vs. the current situation, and were compared with the nominal gross domestic product per capita in each country [39] as recommended by WHO [40]. Given the uncertainty in the effectiveness of in-house broiler chicken food chain interventions to control human Campylobacter infections, a sensitivity analysis of the investigated Campylobacter control strategy (consisting of currently available food safety technologies with good consumer acceptance) was also conducted, selecting the lowest built-in effectiveness options for the selected interventions.
Results
The calculated effect of the EU-wide implementation of the investigated Campylobacter control strategy (consisting of currently available food safety technologies with good consumer acceptance) is summarised in Table 3. Even though the adapted model assumptions were applied to the health and economic burden of human campylobacteriosis, this strategy would still result in an annual €60.4 million cost savings and ~26 400 QALY gain at the EU-27 level, preventing about 1.7 million human GE cases each year. In 15 of the EU-27 countries, health benefits and net cost savings could be achieved at the same time. The highest health benefits would be expected in Romania, Poland and Spain (altogether 51% of the EU-27 QALY gain), whereas most of the expected cost savings would occur in Spain and France (two-third of the net EU-27 cost savings). In most countries without cost savings, the incremental cost-effectiveness ratio would remain well below the nominal GDP per capita, except for Finland, Sweden and Denmark with ICER values 4.42, 2.10 and 1.04 times higher than the GDP per capita in these countries, respectively. The relatively poor cost-effectiveness of the investigated strategy in Finland reflects the very low health gain in this country (due to low baseline incidence of Campylobacter GE) and net annual costs of the intervention strategy of about €0.5 million.
Table 3. Calculated annual impacts of the EU-wide implementation of a Campylobacter control strategy consisting of all currently available food safety technologies with good consumer acceptance

Farm-level and slaughterhouse-level testing points are included. Comparator: current situation. CoI, cost of illness; ICER, incremental cost effectiveness threshold (Δcost/ΔQALY). Negative ICER/GDP per capita values indicate cost savings with health benefits.
The calculated benefits of bacteriocin treatment (together with testing points 1 and 2, see Fig. 1) under the same modelling assumptions are summarised in Table 4. Accordingly, bacteriocin treatment could prevent about 1.8 million human campylobacteriosis cases each year, resulting in 27 364 annual QALY gain and €131.6 million annual net cost in the EU-27. The highest health benefits would be expected again in Romania, Poland and Spain. Any cost savings would occur in Spain and Bulgaria, whereas 80% of the total EU-27 cost would be concentrated in the UK, Italy, Poland, Germany and the Netherlands. ICER values more than three times higher than the per capita GDP would be expected in Finland (12.75), Sweden (8.16) and Estonia (3.73). The net annual costs of the bacteriocin strategy in these countries would be ~€5.5 million in total. The same numbers are expected for vaccination, since the model assumes identical costs and effectiveness of these two alternative control measures [20, 21].
Table 4. Calculated annual impacts of the EU-wide implementation of bacteriocin use as a Campylobacter control strategy in broiler chicken

Farm-level and slaughterhouse-level testing points are included. Comparator: current situation. CoI, cost of illness; ICER, incremental cost effectiveness threshold (Δcost/ΔQALY). Negative ICER/GDP per capita values indicate cost savings with health benefits.
Results of the sensitivity analysis of the Campylobacter control strategy consisting of currently available food safety technologies with good consumer acceptance are summarised in Table 5. Even though the lowest built-in effectiveness options are applied, the model suggests that about 1.3 million human campylobacteriosis cases could be prevented by this strategy in each year, with an annual health benefit of 20 523 QALYs and a net annual cost saving of €17.8 million at the EU-27 level. ICER/GDP per capita ratios by countries are in the range of −0.37 to 1.51, except for Finland (5.89) and Sweden (2.80; Table 5).
Table 5. Sensitivity analysis: calculated annual impacts of the EU-wide implementation of a Campylobacter control strategy consisting of all currently available food safety technologies with good consumer acceptance, assuming low intervention effectiveness

Farm-level and slaughterhouse-level testing points are included. Comparator: current situation. CoI, cost of illness; ICER, incremental cost effectiveness threshold (Δcost/ΔQALY). Negative ICER/GDP per capita values indicate cost savings with health benefits.
Discussion
Evaluation of the investigated Campylobacter control strategy
Human campylobacteriosis attributable to broiler chicken consumption is in the forefront of national- and EU-level food safety policy research, which is presently at the stage of quantitative modelling of health and economic impacts of the implementation of potential control measures. The recently developed ‘Model on Campylobacter control measure costs and effects across EU’ application [20] as adopted by the EC provides country-level cost and health impact estimates for selectable scenarios of farm-level and slaughterhouse-level control measure options, two proposed testing points on farm and on slaughter, and their combinations, along with adjustable assumptions on intervention costs and effectiveness and broiler industry structure in the selected countries. The development and unrestricted availability of this model application is an important step towards evidence-based decision-making by food safety managers and policy-makers in the EU and in the MSs and stimulates further research in this important field. Since model results always strongly rely on the appropriateness of model assumptions, the independent confirmation of the modelling approach will boost further confidence in the model conclusions. We conducted an independent review of two key model assumptions, the health and economic burden of human campylobacteriosis, both without selectable alternative values in the published model. Adapting the fixed cost of illness estimate by country-specific correction factors as proxies of the cost of productivity loss and of the general level of direct healthcare costs in the specific MSs, we found that the single fixed estimate would clearly overestimate the more realistic, country-specific cost of illness estimates in a large part of the EU, representing about 75% of human campylobacteriosis cases. Assuming the same fixed healthcare and economic cost per human campylobacteriosis case from Western to Eastern and from Northern to Southern EU MSs is certainly unrealistic, and the applied country-level adaptation is an important step forward to appropriate model input data, even though our proxy-based approach was not validated by local micro-costing studies. In addition, the 38.9 DALYs per 1000 human Campylobacter GE cases of the published model also seemed to overestimate the health burden, since no discounting was applied on the health burden (in contrast to the 4% annual discount rate on capital). Moreover, the assumed health burden in the original model encompassed inflammatory bowel diseases and post-infectious irritable bowel syndrome, two conditions raised as potential long-term sequelae of acute Campylobacter GE but without convincingly proven causal associations to Campylobacter [12]. Accordingly, the latter conditions were omitted in our critical reassessment, in agreement with the recent update of the Campylobacter disease outcome tree in the USA [Reference Hoffmann, Batz and Morris23, Reference Batz, Hoffmann and Morris24] and with some European papers [Reference Mangen11]. Our adapted European campylobacteriosis burden per case estimate is numerically consistent with the range of previously published estimates, as long as the burden of inflammatory bowel diseases and irritable bowel syndrome are not taken into account.
Expressing the health burden in QALYs may be first confusing in the Food Safety Risk Analysis context where health burden is typically expressed in DALYs. However, the health burden is occasionally expressed in QALYs also in this field [Reference Hoffmann, Batz and Morris23, Reference Batz, Hoffmann and Morris24, Reference Shin41]. Our suggestion on the preferred use of QALY as the universal health metric in Food Safety Risk Analysis is not about the (typically slight) numeric difference between DALY and QALY health burden estimates, but about the direct comparability of food safety control measures to alternative health technologies within the Health Technology Assessment paradigm. In cost-utility analyses of health technologies, health utility is almost always measured in QALY. Thus, expressing the health impact of food safety measures also in QALYs could support the positioning of food safety interventions as a specific class of health technologies, with two important consequences. First, food safety control measures that are shown to be cost-effective in the Health Technology Assessment paradigm could potentially be co-financed by healthcare payers, providing additional financial resources for Food Safety Risk Analysis. Second, the allocative effectiveness of healthcare systems might potentially be further improved this way, investing public resources into those food safety control measures that are clearly more cost-effective than the alternative health technologies [Reference Pitter26]. This approach could be a move towards better integration of these currently hardly connected public policies, as envisioned in the ‘One Health’ concept [Reference Zinsstag42].
Using the adapted health and economic burden parameters, we calculated the expected health and economic impact of the EU-27-wide implementation of a control strategy consisting of all currently available control measures with good consumer acceptance, together with the corresponding testing activities. According to the original model, this strategy was predicted to prevent 1.7 million Campylobacter GE cases each year with an annual cost savings of €353 million and an annual health benefit of 67 328 DALYs averted. Our critical reassessment resulted in remarkably lower anticipated cost savings and numerically lower health benefits; nevertheless, it was confirmed that this strategy is expected to generate substantial health benefits and cost savings at the EU-27 level. Considering the individual EU MSs, this control strategy seems to be dominant (cost savings with health benefits), or at least highly cost-effective (ICER below GDP per capita, as defined by WHO [40]) in all MSs, except for Finland, Sweden and Denmark with minimal net costs and health benefits in these countries, due to low baseline incidence of Campylobacter GE. Vaccination or bacteriocin interventions do not promise a more cost-effective approach in this model and are still under development with remarkable uncertainty about their commercial availability. Accordingly, the available evidence suggests that the implementation of control measures to enhance biosecurity at farms and to improve hygiene at slaughterhouses, together with a thinning ban, is (i) acceptable for the consumers, (ii) would efficiently reduce the disease burden due to Campylobacter and (iii) would be highly cost-efficient or would even generate cost savings in most EU countries. The reader may wonder why this strategy has not yet been extensively implemented after the publication of the original model in 2012. We discuss the putative barriers and their potential solutions in the next section, together with the findings of a sensitivity analysis with lower intervention effectiveness assumptions.
Putative barriers to the implementation of the investigated Campylobacter control strategy
An important barrier to the implementation of the evaluated strategy is probably the fact that economic costs and benefits are separated across multiple systems and budgets. The costs of the interventions are to be paid mostly by the farms and slaughterhouses, while most of the economic benefits would occur in the wider economy (productivity gain) and partly as direct healthcare cost reduction (Fig. 2). Accordingly, private investments and increased production costs at farms and slaughterhouses should be compensated from public resources, recognizing that the implementation of appropriate food safety interventions is in the interest of the greater society. The integrated evaluation of changes in direct healthcare costs and productivity is also a prerequisite for the rapid application of food safety strategies resulting in benefits in both areas. Indeed, the consideration of indirect costs is an established practice in Health Technology Assessment, at least as a sensitivity analysis [Reference Drummond43, Reference Rothermich and Pathak44]; this holistic attitude could also be followed in the food safety paradigm.
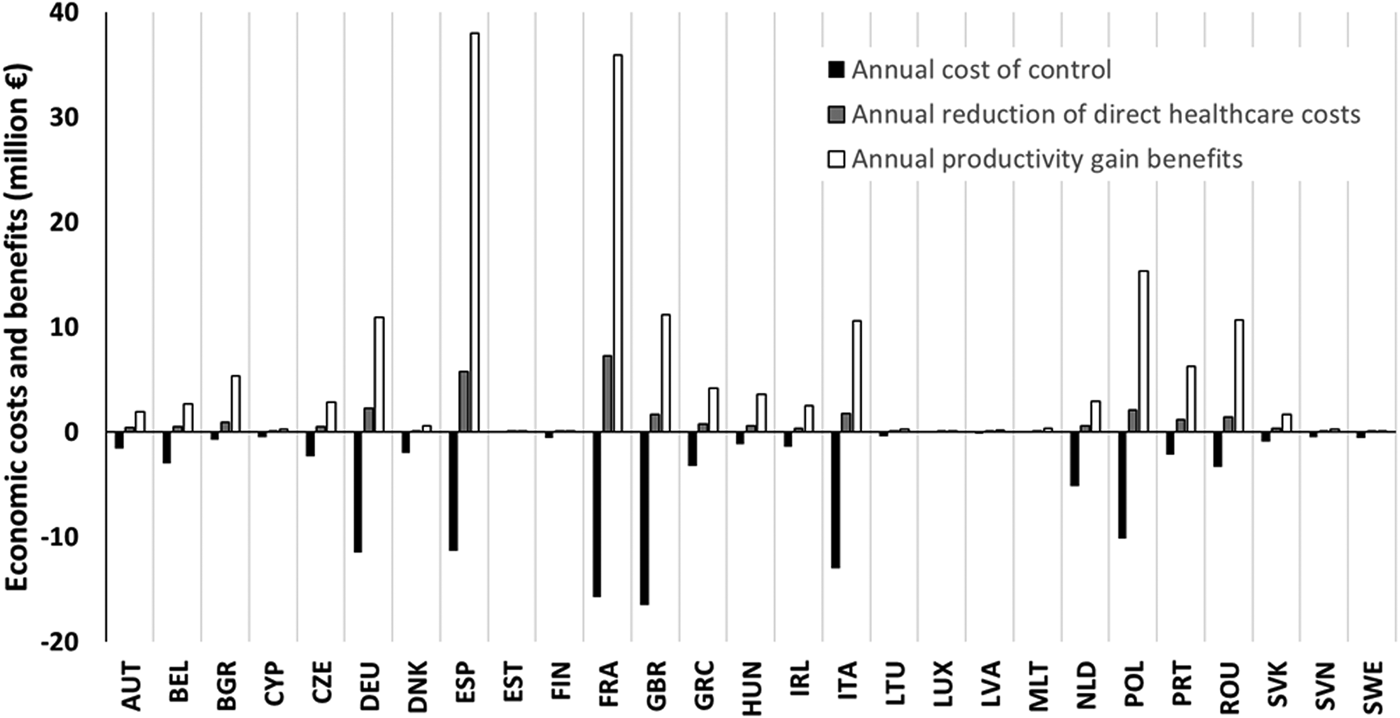
Fig. 2. Composition of annual economic costs and benefits of the investigated strategy (currently available food safety technologies with good consumer acceptance) in the EU-27 Member States, according to the adapted model.
Another potential issue could be the uneven distribution of expected costs and benefits across the EU MSs: countries with the highest required investments may have disproportionally low benefit from a particular food safety programme, potentially resulting in their insufficient commitment. However, all EU-27 countries could be considered as winners in the case of the investigated Campylobacter control strategy, except for Finland where this strategy would not be cost-effective along the WHO definition (ICER above three times the GDP per capita [40]). The low baseline Campylobacter incidence in the broiler sector in Nordic countries would allow a country-specific waiver for Finland in this specific case. As a more general solution, food control interventions could be supported from centralised EU funds in justified cases.
A further important concern could be the inherent uncertainty in model input data and hence, in model conclusions. Input parameter entry fields and model results are provided as point estimates without confidence intervals in the available model; therefore, the uncertainty in the expected costs and benefits is of unclear magnitude. Deterministic sensitivity analyses are allowed by built-in model features for intervention costs and effectiveness, and from the technical point of view, even the fixed model parameters can be substituted with alternative values, as we have approached it in this paper for two important input parameters on the related health and economic burden. Nevertheless, there are also several limitations in the adapted model in this context. Intervention effectiveness assumptions are typically based on a few small-scale studies with unclear external validity. As an example, on-farm biosecurity measures were assumed by the model to result in 40%/55%/70% reduction in broiler colonisation (selectable options in the model) based on data from 39 flocks investigated in 2001 in the UK [Reference Gibbens27]. However, a recent multivariate regression analysis on risk factors for Campylobacter colonisation of indoor broiler flocks found remarkable between-country differences in the importance of certain on-farm biosecurity risk factors (presence of anteroom/barrier, e.g. door or low wall, downtime, drinkers, age of house, designated tools by houses, outdoor temperature in the month of slaughter and country effect) [Reference Sommer, Nauta and Rosenquist45, Reference Sommer46]. Based on the current occurrence of these risk factors and their elimination feasibility, the authors estimated only a minor expected effectiveness for most on-farm biosecurity interventions in most countries if implemented alone [Reference Sommer, Nauta and Rosenquist45]. An exception could be the building of new birdhouses in Spain (~50% reduction in flock prevalence) and also in the UK and the Netherlands with an expected ~30% flock prevalence reduction. Interactions across some risk factors were also apparent and statistically significant, indicating that the components of a biosecurity control strategy shall be careful selected in combination, adapted to the country settings [Reference Sommer, Nauta and Rosenquist45]. A limitation of this multivariate regression analysis was that the source questionnaire did not cover the training practices of the investigated farms, although staff education and compliance is obviously a critical issue when biosecurity improvement is approached [20]. Accordingly, the UK-based biosecurity effectiveness assumption in the reassessed model was not replaced by more relevant and country-specific effectiveness estimates from that work.
Further limitations of the reassessed model input data include that due to the lack of country-specific data, the same disease outcome probabilities were applied in all EU MSs; the country-specific cost per case estimates have not been validated by direct cost determination exercises; health burden of lethal cases was not corrected for differences in life expectancy; international trade aspects (export and import) were not included in the assessment; and the validity of further model assumptions was not investigated. Ultimately, ideal point estimates and probability distributions of model input parameters are mostly unidentified, may be country-specific and may change over time [Reference Mangen8, Reference Kemmeren9, Reference Mangen11, Reference Gellynck36, Reference Sommer, Nauta and Rosenquist45, Reference van Wagenberg and van Horne47]. In response to these challenges, further intensive research could be suggested to gather country-specific data on disease incidences attributable to Campylobacter infections, on clinical outcome probabilities, on direct and indirect costs of the diseases and their treatment, and especially on the real-world uptake and effectiveness of control measures in all EU-27 MSs. Once appropriate country-specific model input parameters are generated, their regular update would demand further continuous research. Instead of such a sophisticated but time-consuming and expensive approach, the authors propose a more pragmatic way based on risk-sharing schemes, similarly to the established practice in the Health Technology Assessment paradigm [Reference Garrison48–Reference Klemp, Fronsdal and Facey50]. In this approach, a residual uncertainty is accepted, and the corresponding risk is shared among the relevant stakeholders. For the investigated Campylobacter control strategy, a potential risk-sharing scheme might include the public funding of national pilot studies on intervention effectiveness to adapt and fine tune the proposed control strategy; public co-payment for the control measures at farms and slaughterhouses; performance-based co-payments of farmers and slaughterhouses to achieve and maintain their commitment to the control measures; and budget control instruments (e.g. volume limits for public co-payment) to protect the public payers against the abuse of public funding. Such a risk-sharing framework is more and more frequently used in Health Technology Assessment for pharmaceuticals and could also help the smooth introduction of food safety interventions.
Finally, the old-style nature of the currently available control measures with good consumer acceptance may also constitute a barrier of implementation. Enhanced biosecurity at farms in practice includes the changing of footwear and clothing of workers when moving between birdhouses, supported by appropriate changing facilities and staff training. Best practice hygiene at slaughterhouses also relates to the well-recognised importance of sanitation in the food industry. These control measures might be seen as old-style techniques by some stakeholders when compared with scientifically more appealing options under development, e.g. bacteriocin treatment or vaccination of the animals. Nevertheless, the adapted model suggests that bacteriocin treatment or vaccination would be far less cost-effective than the combination of currently available and acceptable control measures, and the number of prevented Campylobacter GE cases by these novel methods would only be marginally higher (Tables 3 and 4). It should also be stressed that the interventions analysed here only have a significant effect if applied together, along the food chain. None of the interventions is able to totally eliminate the microbe from the broiler production system, and Campylobacters will cross-contaminate raw materials, processing environment and food products at a later stage of the chain if the interventions are not applied or are not applied correctly at those steps. One could also argue that the importance of hygiene in the food sector has been so widely accepted and well known for decades that no relevant improvement could be expected in this aspect. However, the proposed risk-sharing scheme including the co-payment of farmers/slaughterhouses upon low performance could achieve the breakthrough in the preventive effectiveness of these well-established control measures, as assumed in the published model [20, 21].
Our conceptual overview of putative barriers is based solely on theoretical and economic considerations, and legal aspects were not investigated. This work will support further research on stakeholder perceptions on the realistic barriers and solutions. Notably, the investigated Campylobacter control strategy would be effective, readily available, would generate both cost savings and health benefits at the EU level, and would be highly cost-effective in almost all EU MSs as compared with the current practice. Accordingly, its implementation would meaningfully contribute to the development of European food safety and public health. As a next step, a real-world barrier analysis is proposed, involving all relevant stakeholder groups in the EU.
Conclusions
The original input parameters of the published model overestimated the health and economic burden of human campylobacteriosis in the EU. A more realistic estimate of human campylobacteriosis-related health burden was proposed as 15.23 QALYs in 1000 Campylobacter GE cases. Country-specific correction factors were developed to adjust the cost of illness per case estimate to various countries. The investigated Campylobacter control strategy (combination of all currently available control measures of good consumer acceptance) was reassessed with these adapted input parameters and its high cost-effectiveness was confirmed, even in a sensitivity analysis selecting the lowest built-in intervention effectiveness options of the model. Bacteriocin treatment or vaccination of the animals were also evaluated as alternative control measures, and these strategies seemed to be far less cost-effective and only marginally more effective than the investigated strategy. Putative barriers to the rapid implementation of the investigated Campylobacter control strategy include the uneven distribution of costs and benefits across stakeholders and across MSs, and the inevitable uncertainty in input parameters and model conclusions with concerns about intervention effectiveness and real-world uptake. Potential solutions to these putative barriers including public co-funding of safety measures through risk-sharing schemes across stakeholders, and country-specific waivers for countries with very low incidence and expected poor cost-effectiveness of the selected control strategy were proposed. Further research is required on the stakeholders’ perspectives on the real-world barriers and opportunities for Campylobacter control measure implementation in the indoor broiler chicken food chain.
Acknowledgements
The authors express their special thanks to Mr. Kirk Lederhaas for linguistic proof-reading of the manuscript.
Conflict of interest
None.









