INTRODUCTION
Rotavirus is the main cause of diarrhoea-related illness and death in children worldwide. It is associated with more than 2·3 million hospitalizations and up to 600 000 deaths in children aged <5 years [Reference Parashar1]. In Australia about 20 000 children aged <5 years are admitted to hospital with acute gastroenteritis each year, with rotavirus being responsible in ~50% of cases [Reference Galati2]. The direct medical costs in Australia are estimated at over Aus$30 million annually [Reference Galati2].
Rotavirus infection usually results in acute gastroenteritis characterized by vomiting and severe watery diarrhoea in >90% of cases and fever in 30% [Reference Beers and Berkow3], often leading to dehydration. Children usually become infected in the first 3–5 years of life with the highest incidence in those aged <2 years [Reference Galati2].
Acute gastroenteritis is a common reason to seek medical attention. A general practitioner consultation is sought in 75% of cases, advice from accident and emergency departments in 50% of cases and from pharmacists in 5% of cases [Reference Elliott, Backhouse and Leach4]. The mean cost of hospitalization for rotavirus infection is estimated at about Aus$2160 per child and the cost of care in the community at Aus$550 per child [Reference Liddle5]. Together with the added costs of missed work and schooling, rotavirus places a large financial burden on the health system and society [Reference Liddle5].
Naturally acquired rotavirus infection, protects against subsequent severe disease or re-infection, particularly following repeat exposures [Reference Velazquez6]. After a single natural infection 40% of children are protected against subsequent infection, 75% are protected against diarrhoea caused by subsequent infection and 88% are protected against severe rotavirus diarrhoea [Reference Velazquez6]. Second, third and fourth infections provide progressively greater protection. Immunization with oral attenuated rotavirus vaccines aims to duplicate natural protection and prevent moderate to severe disease, using a multi-dose schedule in the first 6 months of life.
Following the withdrawal of an effective rotavirus vaccine licensed in the United States in 1998 due to an association with intussusceptions [Reference Murphy7], two live attenuated rotavirus vaccines have been tested in large-scale randomized controlled trials [Reference Ruiz-Palacios8, Reference Vesikari9]. These two live attenuated oral vaccines were licensed for use in Australia in 2006 and are now funded on the National Immunization Program (NIP) for all Australian infants.
The aim of this study was to collect detailed data on the burden, management and outcomes of rotavirus infection in children aged <5 years who presented to a tertiary paediatric hospital in Australia.
METHODS
All stool samples that were tested for rotavirus antigen by EIA (Vidas; bioMérieux, Marcy l'Etoile, France) in 2004, at the Children's Hospital at Westmead [a tertiary-care paediatric hospital in Sydney, New South Wales (NSW)], were identified from microbiology department records. Medical records were reviewed for those children aged <5 years with a positive or equivocal result who presented to the Children's Hospital at Westmead emergency department or who were in-patients. Data were extracted onto a standard form.
Demographic information on sex, age at presentation and country of birth was collected. Immunization status of patients was assessed as ‘up-to-date’ or incomplete, according to the Australian Standard Vaccination Schedule [10]. The study was conducted prior to the availability of rotavirus vaccines. Children were classified as either breastfed, formula-fed, or if the child was in an older age group (>18 months), this was recorded as not applicable. Date of presentation, onset and duration of symptoms and admission were determined for each patient. The presence of symptoms and signs commonly associated with rotavirus gastroenteritis including diarrhoea, vomiting, fever, lethargy and dehydration were recorded as well as weight loss, a decrease in oral intake and any other symptoms experienced. It was determined whether rotavirus infection was nosocomial (symptoms occurred 3 days after admission for an unrelated illness) or community acquired and whether the child was admitted as an in-patient. Total admissions to the Children's Hospital at Westmead in 2004 (26 029) were obtained. The rate of nosocomial infection per 10 000 admissions was determined.
The number of investigations performed, including blood tests, microbiological culture, radiology and other tests were obtained along with details of medical management. Where stated, it was determined if other health professionals had been consulted prior to presentation and whether any management had been performed at home.
Principal and comorbid diagnoses were verified by checking against the ICD-10-CM [11] coding on discharge. A number of patients who were not assigned official ICD codes were classified with the provisional diagnosis of ‘gastroenteritis, viral’ as assigned on the emergency department record.
Descriptive, univariate analysis was done using SAS software (SAS Institute, Chicago, IL, USA). The study was approved by the Children's Hospital at Westmead Research Ethics Committee (MR 2005-06-07).
RESULTS
A total of 1268 stool samples of children were tested for rotavirus at the Children's Hospital at Westmead in 2004. Eighty-six (7%) children tested positive for rotavirus. Five patients were excluded from the study as they aged >5 years. In one case the same patient tested positive twice on a single admission and was therefore only counted once. The records of 80 patients were reviewed. An additional 39 children had an equivocal result; however, these records were not included in the main analysis.
Figure 1 shows that compared to previous years, the number of positive tests in 2004 was lower. The most cases of rotavirus gastroenteritis were identified in the months of September and October. In 2004 the greatest number of cases occurred in September with 26 positive stool test results.

Fig. 1. Stool samples testing positive or equivocal for rotavirus at the Children's Hospital at Westmead, January 2000 to December 2004.
The age distribution of children with rotavirus is shown in Figure 2. About half of the children (39/80) in the study were aged <12 months and 75% (62/80) were <2 years. The mean age of children with rotavirus-positive stool samples was 13 months. Sixty-one (61%) of the patients were male and thirty-one (39%) female. Ninety percent of children (72 patients) were born in Australia. Only 15 (19%) patients reported contact with others with gastrointestinal symptoms prior to presentation. Immunization status was reported as up-to-date in 75% of cases, incomplete in 18% of patients and in 8% immunization status could not be determined. In those infants in whom the method of feeding was recorded: five children were breastfed at time of presentation compared to 31 formula-fed children.

Fig. 2. Age of 80 children aged <5 years with positive rotavirus stool tests presenting to the Children's Hospital at Westmead, 2004. ■, Male; □, female.
Rotavirus infection was community acquired in 69/80 patients (86%). Nosocomial infection was reported in eight patients (10%) during their hospital admission for causes other than rotavirus gastroenteritis; acquisition was unknown for three patients. Of the 26 029 admissions to the Children's Hospital at Westmead in 2004 nosocomial rotavirus infection occurred at a rate of 3/10 000 hospital admissions. Of the nosocomial cases, four (50%) were aged <12 months. Of those children who were became infected with rotavirus whilst in hospital, the most common presenting symptoms were vomiting (88%), diarrhoea (75%), and fever (63%) with a mean symptom duration of 1·5 days. The principal diagnoses for in-patients who acquired rotavirus during their stay included conditions such as encephalitis, generalized epilepsy and other convulsions. Many of these patients had multiple comorbid diagnoses.
Of the 69 patients who acquired rotavirus in the community, all but one presented to the emergency department. Twenty-six (38%) of those children presenting to emergency were admitted to hospital and the remainder (62%) were treated and discharged from the emergency department. However, of all patients only 27 patients (39%) were discharged within 24 h, with the remainder requiring one or more nights in the hospital or emergency department (Fig. 3). In general, admissions were short with most only requiring 1–3 days as an in-patient (Fig. 3).The mean length of stay for those acquiring rotavirus infection in the community was 2·3 days.

Fig. 3. Length of stay in hospital for 69 children with community-acquired rotavirus presenting to the Children's Hospital at Westmead in 2004 (0 day represents children admitted for <24 h).
In children aged <2 months who acquired rotavirus infection in the community the mean length of stay was 1·5 days compared to 3·0 days in those aged ⩾12 months. Of children aged <12 months, 10/33 (30%) patients were admitted to hospital and the remainder were discharged from emergency.
Most children presented with symptoms of diarrhoea, vomiting and fever (Table 1). Dehydration affected 39% of children. Many patients presented with other symptoms, which included respiratory symptoms, rash and febrile seizures.
Table 1. Presenting symptoms of 69 children testing positive for community-acquired rotavirus gastroenteritis, who presented to the Children's Hospital at Westmead in 2004

All 80 patients in the study had their stools tested for rotavirus and a stool culture performed for bacterial pathogens. For 20 children these were the only tests that were performed. In total, 599 investigations were performed. The frequency and number of investigations are presented in Table 2. The median number of investigations per patient was 4 (mean of 5·5), excluding stool culture and rotavirus antigen testing. Common investigations included assessment of urea, electrolytes and creatinine (UEC), full blood count (FBC) and blood culture. Eleven children required a lumbar puncture and assessment of cerebrospinal fluid as part of a septic work-up.
Table 2. Investigations performed in 80 children that tested positive for rotavirus gastroenteritis at the Children's Hospital at Westmead in 2004Footnote *
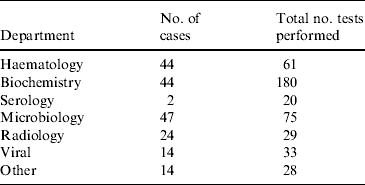
* Excludes stool culture and rotavirus antigen testing.
Management for rotavirus gastroenteritis included rehydration with oral rehydration solution (ORS) and intravenous (i.v.) fluids and the prescription of antibiotics and other medications including paracetamol (Table 3). Thirty-seven children (46%) received i.v. fluids, most commonly N/2 saline and 2·5% dextrose. Six children received a bolus of normal saline (NS). ORS was given to many patients in the waiting area of the emergency department prior to consultation. A total of 39 (49%) children received ORS, in the form of either gastrolyte or hydrolyte. Five patients were rehydrated with ORS via nasogastric tube.
Table 3. Management performed in 80 children that tested positive for rotavirus gastroenteritis at the Children's Hospital at Westmead in 2004
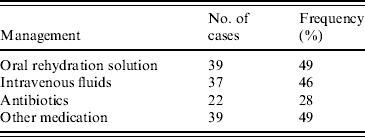
Antibiotics were prescribed to 22 (28%) patients. The most frequently used antibiotics were cefotaxime, ampicillin and gentamicin. Nine patients received cefotaxime, four had ampicillin, gentamicin or both and three patients were prescribed EES (erythromycin ethyl succinate). Other antibiotics given included; timentin (1 patient), benzyl penicillin (2), vancomycin (1), cephalexin (3) and rifampicin (1). Two patients received the antiviral acyclovir. Thirty-nine patients (49%) were given other medication of which paracetamol was the most common followed by ibuprofen. Two patients received phenobarbitone and five patients presented with the need for salbutamol.
Of all 35 patients managed in hospital, 24 (69%) received i.v. fluids, 20 (57%) received antibiotics and 29 (83%) received other medications. This contrasts with those 43 patients not admitted to the ward where 13 (30%) received i.v. fluids, two (5%) received antibiotics and 10 (23%) received other medications. Fourteen children aged <12 months (36%) were treated with i.v. fluids, 19 (49%) received ORS, 10 (26%) were given antibiotics and 16 (41%) other medications. Of those that were breastfed 60% were managed with i.v. fluids. No breastfed children received antibiotics. No patients required admission to the intensive care unit (ICU) due to rotavirus infection.
Only 12/69 children (17%) with community-acquired infection had management performed at home prior to presentation to hospital. Eleven patients were treated with paracetamol. ORS was given in six cases and 14 (20%) saw a health professional prior to presentation. Two children were prescribed the anti-emetic metoclopromide and one promethazine hydrochloride. Seven patients were taking antibiotics at the time of presentation. In general this was cotrimoxazole at prophylactic doses.
Treatment on discharge was recommended for eight patients. Four patients were prescribed cephalexin for urinary tract infection and three children were changed to a lactose-free formula. Most patients were advised to follow-up with their local general practitioner for review of symptoms and stool analysis. One patient was referred for a small bowel biopsy.
The ICD-10-CM codes for each patient were determined from medical records. Principal diagnostic codes are given in Table 4. The code for rotavirus enteritis (A080) was the principal diagnosis in only 23/80 cases. Those without any official coding (28 patients) were patients with a short stay in the emergency department only. Twenty-six of these patients received a diagnosis of ‘gastroenteritis, viral’ in the emergency department.
Table 4. Frequency of principal ICD-10-CM codes for 80 children that tested positive for rotavirus gastroenteritis at the Children's Hospital at Westmead in 2004
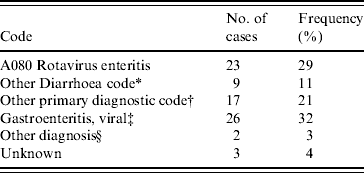
* Other diarrhoea codes include: A09 Diarrhoea and gastroenteritis of presumed infectious origin; A084 Viral intestinal infection, unspecified; R11 Nausea and vomiting.
† Where primary diagnostic code did not include A080, A09, A084, R11.
‡ Primary diagnosis assigned in emergency department where record has not been coded with ICD-10.
§ Diagnosis made, no ICD-10 code.
There were 17 patients with a principal code other than rotavirus enteritis or other diarrhoea. Of those with a code other than rotavirus or infectious diarrhoea as the principal diagnosis, seven (9%) had rotavirus enteritis as a comorbid diagnosis and two (2·5%) a non-specific gastroenteritis diagnosis. Nine patients had multiple diagnoses. Two patients had comorbidities resulting in immunosuppression. These included acute lymphoblastic leukaemia in remission and a liver transplant patient.
Discussion
This study demonstrates the burden and outcome of rotavirus infection in children aged <5 years presenting to a tertiary paediatric hospital. Over half of the children in this study were aged <12 months and 75% were <24 months. The high burden of infection in children aged <2 years is consistent with patterns reported in Australia and other developed countries [Reference Galati2, Reference Ferson12–Reference Newall15]. A greater number of infections occurred in males than in females [Reference Newall15]. The Australian Rotavirus Surveillance Program reported that 72% of positive rotavirus stool samples serotyped from centres around Australia from 1 July 2003 to 30 June 2004 were from infants aged <24 months [Reference Kirkwood16].
While rotavirus gastroenteritis causes up to an estimated 611 000 deaths worldwide [Reference Parashar17], very few deaths occur in developed countries [Reference Parashar1]. In this study, there were no deaths or ICU admissions associated with rotavirus infection, however, it is estimated that about one death occurs annually in Australia from rotavirus infection [Reference Kirkwood16].
The average length of stay for children acquiring rotavirus infection in the community was 2·3 days, similar to previous findings [Reference Galati2, Reference Liddle5]. Galati et al. [Reference Galati2] suggested that shorter length of stay in Australia, compared to that seen in the United States (3·9 days) [Reference Jin18], may reflect a less severe spectrum of illness, better management or more widespread use of ORS. This study involved only children who presented to hospital and whose stools were tested for rotavirus and therefore may represent those with more severe disease.
The high use of i.v. fluids (46%) and antibiotics (28%) may also reflect an increased severity of cases and dehydration status. Antibiotics would have been prescribed in infants and young children who were febrile, where the clinical assessment suggested a possible bacterial infection. There were also a relatively large number of children [11] who received a lumbar puncture, which is a diagnostic test often performed in young infants with fever and vomiting. The extensive investigations may have resulted from children presenting with general systemic symptoms of sepsis and consequently having a full septic work-up.
The use of ORS use prior to hospital presentation was low, although 49% of children in the study were given ORS after presenting to hospital. It has been proposed that increased use of ORS would minimize the need for hospitalization, use of i.v. fluids, antibiotics and anti-diarrhoeal and anti-emetic agents [Reference Elliott and Dalby-Payne19]. ORS has been shown to be as effective as i.v. fluids in treating children with mild to moderate dehydration with fewer side-effects [Reference Gavin, Merrick and Davidson20, Reference Fonseca, Holdgate and Craig21]. In NSW management of gastroenteritis in infants is directed by clinical practice guidelines published by the NSW Department of Health [22]. Oral rehydration via oral or nasogastric fluid is the recommended route for cases of mild to moderate dehydration. Use of anti-vomiting, anti-motility or anti-diarrhoeal agents are not recommended and the administration of antibiotics is only supported in certain bacterial cases following microbiological advice. Measurement of UEC, blood sugar level (BSL) and FBC are recommended for dehydration >5%, with blood cultures if the temperature is >38·5°C.
Few Australian studies of nosocomial rotavirus infection are available. We found 10% of rotavirus infections were nosocomially acquired. Higher rates have been reported in other countries, including the United States (21%) [Reference Rodriguez-Baez23] and Ireland (23%) [Reference Harrington, Butler and Cafferkey24]. Nosocomial rotavirus infection prolongs hospital stay and increases hospital costs [Reference Harrington, Butler and Cafferkey24, Reference Gianino25].
The accuracy and completeness of the medical records reviewed is largely unknown. This is a major limitation of the study and the result should be interpreted in this context. In 2004 there were fewer cases of rotavirus infection at the Children's Hospital at Westmead compared to other years. Although it cannot be determined precisely, this did not appear to be associated with changes in patterns of health-care delivery or testing in the hospital, but may have been due to a lower seasonal activity from rotavirus in that winter. Another limitation of this study is that it did not take into account those children presenting with symptoms of gastroenteritis whose stools were not tested, consequently a number of children presenting with mild to moderate gastroenteritis may have been excluded.
This study shows that a low percentage of patients with laboratory-confirmed disease had rotavirus enteritis as a primary diagnostic code (29%). This could lead to an underestimation of the incidence of rotavirus in reported statistics that rely on ICD-10 coding. A similar trend was reported by a study in the United States that evaluated the sensitivity of the rotavirus ICD code among children hospitalized for acute gastroenteritis [Reference Hsu26]. In this study only 38% patients with laboratory-confirmed rotavirus disease were assigned a rotavirus code [Reference Hsu26].
The rotavirus vaccine has recently been added to the NIP for all Australian infants. We would expect to see a reduction in the number and severity of cases of rotavirus presenting to hospital with the introduction of universal vaccination [Reference Newall27]. The data collected in this study may further inform the assessment of the rotavirus vaccination programme by providing information on the burden, management and outcomes of rotavirus disease.
ACKNOWLEDGEMENTS
A. T. N. was funded by a Public Health Postgraduate Scholarship (402920) from the National Health and Medical Research Council (NHMRC) of Australia, and by a GSK Support Grant. C.R.M. has had investigator-driven research funded by GSK and CSL/Merck, but this research did not involve rotavirus.
DECLARATION OF INTEREST
None.









