Introduction
In the USA, influenza is a major cause of medically attended acute respiratory illness (MAARI)-related pediatric hospitalisations [1]. From 1993 to 2008, the estimated rate of influenza-associated hospitalisations was 91.5 per 100 000 for children less than 1 year of age and 21.9 per 100 000 for children ages 1–4 years [Reference Zhou2]. Information about pediatric admissions to intensive care units (ICUs) and mechanical ventilator use during seasonal influenza outbreaks is scarce, particularly for children of all ages.
The primary objective of this study was to examine whether surges in pediatric MAARI-related hospitalisation, ICU admission, or mechanical ventilation, were associated with influenza outbreaks. We examined this association in all 50 State of Maryland's acute care hospitals for 12 consecutive seasons.
Materials and methods
Periods of interest for each study season
We used MMWR weeks defined by US Morbidity and Mortality Weekly Report (MMWR) to delineate each of 12 consecutive study seasons from 2001 to 2013 [3]. A study season was defined as the beginning of US Center for Disease Control and Prevention (CDC) week 27 (late June or early July) of 1 year to the end of week 26 (late June or early July) of the subsequent year so as to include all influenza outbreaks for each study season. An aberration of note was study season 2008–2009, which ended on 4 July 2009 (the end of week 26). This study season contained an influenza outbreak starting in January of 2009 and ending in April of 2009 and also captured the first wave of the 2009 A/H1N1 pandemic virus outbreak, which began in April 2009 and ‘ended’ in early in July of 2009.
The study season 2009–2010 started on 5 July 2009 (the beginning of week 27), and ended on 4 July 2010 (the end of week 26) and included the second wave of the A/H1N1 pandemic virus in the fall of 2009.
The influenza outbreak period (IOP) for each of the 12 study seasons was defined using a pool of anonymous weekly positive influenza test results obtained from an interactive CDC website [4] collected for US Department of Health and Human Services (HHS) Region 3, which contains Maryland, Pennsylvania, Delaware, Virginia, West Virginia and the District of Columbia. These test results were used to define discrete periods for analysis of each study season as visually depicted in Figure 1. Each study season's IOP was defined as the fewest consecutive MMWR weeks containing at least 85% of the positive influenza tests for HHS Region 3, with an added 2-week buffer on either ‘side’ of this interval to ensure we captured most of the influenza activity for that individual season. The peak IOP (PIOP) consisted of the four consecutive MMWR weeks with the highest aggregate number of positive influenza tests, including the peak week. The off-peak influenza period (Off-PIOP) included the IOP minus the 4 weeks of the PIOP. Finally, the non-influenza season (NIS) for each season consisted of the remaining weeks outside the IOP for each study season.

Fig. 1. Example of time periods used in the analysis for study seasons. PIOP, (peak influenza outbreak period) defined as four consecutive weeks with the highest number of positive influenza tests; Off-PIOP, (off-peak influenza outbreak period) defined as IOP minus the central PIOP; NIS, (non-influenza season) represents the weeks in the study year not including IOP.
Study population and medical outcome data
The study population included all children ages 0–17 years admitted to any of Maryland's state-regulated acute-care hospitals between 1 July 2001 and 29 June 2013. Importantly, these hospitals represented a broad spectrum of community, teaching and public acute-care medical centres. In 2014, of the 50 Maryland acute care hospitals 37 reported having 1–140 pediatric beds [5]. Data from newborns admitted to the newborn nursery or neonatal ICU were not included because their illness was not likely to be directly related to influenza. The Health Services Cost Review Commission (HSCRC) of the State of Maryland Department of Health and Mental Hygiene provided all hospitalisation data for children for the 12 consecutive study seasons.
De-identified data extracted from the HSCRC database included each child's admission date, but not discharge date or hospital of admission, age in months for infants or years for older children, gender and race. Additionally, the HSCRC database allowed us to identify three key indicators of medical resource utilisation, namely, hospitalisation (excluding the full-term nursery), admission to a pediatric or medical ICU (excluding a neonatal ICU) and mechanical ventilator use during the hospital stay. Mechanical ventilation use was determined from the HSCRC database using Current Procedural Terminology (CPT) codes for continuous ventilation (96.70, 96.71 and 96.72). Also, MAARI-related deaths during the hospitalisation were identified.
To identify pediatric patients who required advanced medical resources for the management of acute respiratory illness, diagnostic International Classification of Diseases version 9 (ICD-9) codes for MAARI were used in a manner previously described [Reference Lichenstein6–Reference King9]. MAARI-related diagnoses included one or more primary or subsequent ICD-9 discharge codes for upper, middle and lower respiratory illnesses as well as specific codes for fever, respiratory viral illness and influenza.
Statistical analysis
The key analyses examined whether surges for each of the three medical support utilisation indicators for children with MAARI-related diagnoses occurred during the PIOP or Off-PIOP compared with the NIS. This was accomplished using mean daily rates for each of the three medical resource outcome indicators during the PIOP and Off-PIOP and comparing them with mean daily rates of these outcomes during the NIS. A Poisson regression model, allowing for over-dispersion, was used to estimate the rate ratio (RR) and associated 95% confidence interval for all 12 study seasons combined. In addition, the model included a factor for each study season to adjust for seasonal variability likely caused by a variety of factors such as other respiratory pathogens as well as seasonal or environmental issues that could have influenced the utilisation outcomes of interest. The RRs were considered a quantitative indicator of a surge in use of the three advanced medical supports as well as hospital deaths during the PIOP or Off-PIOP compared with the NIS. These RRs were estimated for children of all ages (0–17 years) and for four age-based subgroups: 0 to <5 years, 0 to <2 years, 2 to <5 years and 5 to <18 years.
All data analyses were performed using SAS software (version 9.3, SAS Institute).
Human subject protection
All data regarding influenza surveillance and hospitalisations were examined and analysed without personal identifiers. This study was reviewed and approved by the Institutional Review Board at the University of Maryland, Baltimore, USA.
Results
Demographic information for the study group is presented in Table 1. Approximately 1.36 million children live in the State of Maryland [10]. During the 12 study seasons, there were 388 761 pediatric admissions to any of the state's 50 acute-care hospitals regardless of diagnoses. For all 12 study seasons combined, MAARI-related advanced medical supports were recorded including 100 740 hospitalisations, 12 582 ICU admissions and 3821 mechanical ventilation use. There were relatively few deaths (n = 300) recorded for children hospitalised with MAARI-related illnesses.
Table 1. Demographics of children hospitalised in HHS Region 3 between 1 July 2001 and 29 June 2013
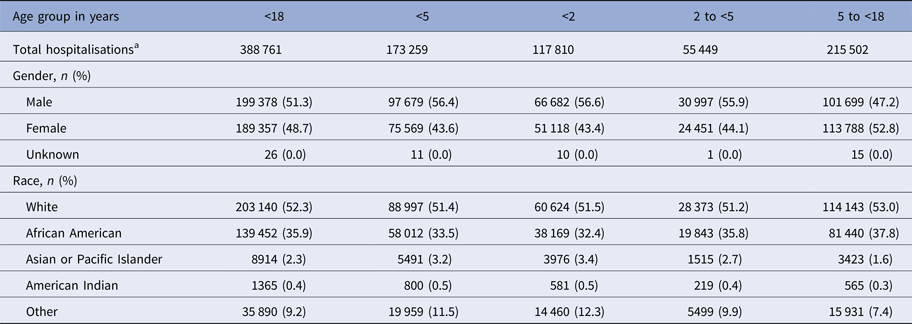
a All-cause hospitalisation for any diagnosis. This designation does not include neonates in the full-term nursery or neonatal intensive care unit.
Measures of the three medical support utilisation indicators and death for children hospitalised with MAARI-related ICD-9 codes are presented in Table 2. This table shows that for the entire pediatric cohort group as well as the four age subgroups, there were significantly greater event rates (rate ratios) of advanced medical support during the PIOP or Off-PIOP compared with NIS for the 12 study seasons combined. However, Table 2 shows there were not significant surges in death for all 12 seasons combined and certainly not for individual study seasons due to the small number of MAARI-related deaths.
Table 2. Rate ratios (RR) for MAARI-related medical outcomes in hospitalised children for all 12 study years

MAARI, medically attended acute respiratory illness.
a All hospitalisations for any diagnoses, This designation does not include infants in the full term nursery or neonatal intensive care units.
b % is calculated by number of medical outcomes divided by N (total number of hospitalisations in that age group with any diagnoses).
c Comparing PIOP vs. NIS based on the Poisson regression model including all 12 study years.
d Comparing Off-PIOP vs. NIS based on the Poisson regression model including all 12 study years.
Figure 2 visually demonstrates the close association of peaks of weekly numbers of the three MAARI-related pediatric medical support items in Maryland acute-care hospitals compared with weekly numbers of positive influenza tests for HHS Region 3, which includes Maryland. These peaks varied from November to March, which further suggests an association of surges of advanced medical support with influenza outbreaks.

Fig. 2. Plots of weekly numbers of MAARI-related medical outcomes (black line) and counts of positive influenza tests (gray bars). MAARI, medically attended acute respiratory illness; Flu Count,weekly numbers of positive influenza tests for HHS Region 3 which includes Maryland.
Discussion
This study clearly demonstrated significant increases in the use of advanced medical support resources, specifically hospitalisation, ICU admission, or mechanical ventilation, for Maryland children with acute respiratory illness during influenza outbreaks. Although we could not confirm whether these children had documented influenza illness from information available in the HSCRC database, the visual temporal association seen in Figure 2 between positive influenza tests and surges in MAARI-related hospitalisation supports the assumption that influenza illnesses were a key factor. Our assumption is further statistically supported by the association of elevated RRs of MAARI-related medical support resources including hospitaliation, ICU admissions and mechanical ventilation use during the PIOPs and Off-PIOPs compared with the NIS. The one exception was the first wave of the pandemic influenza A/H1N1 outbreak that occurred during April to early June 2009. Figure 2 did not show a major surge in medical support for this H1N1 first wave. However, the nadir during the first wave was the lowest during April to June for all the 12 other study seasons. April to June are usually the nadir for more severe viral respiratory illnesses in the USA [Reference Fowlkes11, Reference Stover and Litwin12].
Concerns have been raised regarding the need to increase accessibility to advanced medical care and resources including hospitalisation, pediatric critical care and critical care equipment, during severe influenza outbreaks [Reference Christian13, Reference Toner and Waldhorn14]. On 28 April 2015, the US National Advisory Committee on Children and Disasters (NACCD), at the request of the US HHS, published an assessment of the country's state of readiness for a surge of pediatric patients presenting to medical facilities during large-scale influenza infections or other emerging infectious diseases [15]. The NACCD identified potential gaps in four key areas: (a) adequate staffing by medical personnel, (b) space to accommodate the influx of children, (c) a structured and clearly defined system through which institutions can coordinate successful surge management and (d) availability of age- and size-appropriate medical equipment supplies.
Influenza seasonal outbreaks, as well as pandemics, vary in intensity from season to season [Reference Cox and Subbaro16]. If access to critical care units and equipment is already limited, then even greater shortages of these resources are likely to occur during severe influenza outbreaks. A scarcity of ventilators and associated equipment such as pediatric ventilator circuits, suction catheters and oxygen and carbon dioxide monitors might develop during a severe influenza epidemic or pandemic. Many hospitals have minimal reserves of medical equipment such as mechanical ventilators on site. Therefore, additional units and supplies are often rented from medical supply companies during the course of each season [Reference Christian13, Reference Toner and Waldhorn14]. Federal and state stockpiles of ventilators can be tapped, but these machines might not have the alarm settings or tidal volumes appropriate for pediatric patients [Reference Custer17]. Our study revealed that during influenza outbreaks, a substantial increase in the number of children younger than 2 years of age required ICU admission and mechanical ventilation during MAARI-related hospitalisations.
This study has several limitations. First, infectious outbreaks can be caused by respiratory viral pathogens other than influenza virus such as a respiratory syncytial virus, parainfluenza viruses, adenoviruses, or corona viruses at various times and intensities. Increased use of sensitive respiratory diagnostics may be helpful to examine the impact of concomitant respiratory viral outbreaks, especially respiratory syncytial virus, on advanced medical resource utilisation [18]. Also, HHS Region 3 influenza virus surveillance data from nearby states and the District of Columbia were used to define the IOPs and might not represent the situation specifically in Maryland. Finally, we need to mention that this work is based on observational study data, therefore bias is difficult to avoid and thus possible confounding factors are hard to assess. Despite these limitations, data from this study clearly support the concept that influenza outbreaks are associated with advanced medical support for children with respiratory illnesses.
Conclusion
Our study demonstrated increases in hospitalisations, ICU admissions and mechanical ventilation use temporally related to regional influenza activity over a 12-season period in Maryland. This data reinforces the need for annual vaccination of children of all ages as well as the development of improved influenza vaccines. Our results could also be useful in the development of models to assist in planning for federal, state and local responses to future severe influenza virus outbreaks, including pandemics.
Acknowledgements
The authors wish to thank Oscar Ibarra; Chief of the Information Management and Program Administration for the Maryland Health Services Cost Review Commission (HSCRC) for his assistance in obtaining hospital-based data utilised in this study. No funding was utilised for this study.
Disclaimer and declaration of interest
Note that the views of the authors of this manuscript do not necessarily represent the views of the US Department of Health and Human Resources, including the Food and Drug Administration and Biomedical Advanced Research and Development Authority.
Conflict of interest
All the authors of this manuscript declare that there are no conflicts of interest.
Ethical standards
The University Of Maryland School Of Medicine Institutional Review Board has reviewed and approved this study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






