INTRODUCTION
Globally, acute respiratory infections (ARIs) are the leading cause of childhood morbidity and mortality [Reference Walker1]. ARIs include common cough and cold or upper respiratory tract infection (URI; mild form of disease) and acute lower respiratory tract infection (ALRI; severe form of disease). Children in developing countries experience 3–8 episodes of URI annually [Reference West2]. In 2011, ALRIs were estimated to be responsible for 1·3 million deaths globally in children aged <5 years [Reference Walker1]. In Bangladesh, ARIs are the most frequent cause of health consultations and hospitalizations for children aged <5 years [Reference Azziz-Baumgartner3]. The recent Bangladesh Demographic and Health Survey reported that 5·8% of children aged <5 years experienced ARI [4]. Two studies from rural and urban Bangladesh reported an estimated 0·2–0·5 ALRI episodes/child per year in children aged <5 years [Reference Brooks5, Reference Zaman6].
In the last few decades, researchers have been evaluating the prophylactic and therapeutic use of different micronutrients to reduce infectious disease, including ARI in children [Reference Taylor and Camargo7]. The first studies investigating the role of vitamin D in pneumonia reported an association between nutritional rickets and pneumonia [Reference Lawson8, Reference Najada, Habashneh and Khader9]. Case-control studies conducted later found a significantly lower mean concentration of vitamin D in children with pneumonia than in healthy children [Reference Roth10, Reference Wayse11]. Longitudinal studies reported reduced risk of ALRI in infants, if higher serum vitamin D concentrations were present in cord blood [Reference Mohamed and Al-Shehri12–Reference Camargo14]. A well-designed randomized controlled trial (RCT) performed in Kabul, Afghanistan [Reference Manaseki-Holland15] reported no beneficial effect of vitamin D supplementation on the incidence of pneumonia in children aged <2 years. However, it has been suggested that the lack of an effect of vitamin D reported in previous studies may be due to the confounding effect of supplementation dose, high rates of undernutrition, and the presence of other micronutrient deficiency in study participants [Reference Martineau16].
A longitudinal study reported a greater risk of incidence of ARIs in children with vitamin A deficiency or in children suffering from undernutrition [Reference Pandey and Chakraborty17]. Case-control studies conducted in well-nourished and malnourished children found lower levels of serum zinc in children with pneumonia than in healthy children [Reference Shakur18, Reference Shakur19]. A recent review of nutritional interventions for ALRIs concluded that zinc supplementation can reduce 25% incidence of ALRIs in zinc-deficient populations but vitamin A supplementation beyond the neonatal period does not reduce ALRI incidence [Reference Roth20].
Childhood macro- and micronutrient undernutrition can result in a deterioration of the immune function that can lead to an increased incidence and severity of infectious disease morbidity including ARIs [Reference Rodriguez, Cervantes and Ortiz21]. The studies conducted so far to evaluate the role of vitamin D or vitamin A or zinc in ARIs in children did not consider the nutritional status of children nor other micronutrient status. These limitations warrant the development of longitudinal studies to systematically evaluate the relationship between vitamin D status alone, as well as in combination with other micronutrient status and the incidence of ARIs in underweight and normal-weight children aged 6–24 months. Our analysis of longitudinal data from two parallel cohorts of children (underweight and normal weight), aged 6–24 months from an urban slum of Bangladesh, investigates the association between serum concentrations of vitamin D and ARIs (including URIs and ALRIs), adjusted for socioeconomic factors, retinol and zinc.
METHODS
Study design, site and participants
We used data from an interventional case-control study in an urban slum of the Mirpur field site in Dhaka, Bangladesh, which was one of the field sites of the ‘Malnutrition and Enteric Infections: Consequences for Child Health and Development’ (MAL-ED) research project [22]. The study was approved by the Research Review Committee and the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b).
Descriptions of the MAL-ED study design, site, participants and interventional packages have been reported in detail elsewhere [Reference Ahmed23]. The MAL-ED study conducted biannual surveillance of every household of Bauniabadh area of section 11 of Mirpur to identify eligible participants for the study. In brief, participants (children aged 6–24 months) were screened for eligibility using weight measurement during household surveys. Severe to moderately underweight children (weight-for-age Z score, WAZ <–2·00 s.d.) were considered as cases and controls were well-nourished/normal-weight children (WAZ ⩾–1·00 s.d.) matched for area of residence and sex. Eligible children were invited to participate, and enrolled in the study after their parent/guardian signed the consent form. Overall, 500 cases and 480 controls were enrolled during November 2009 to December 2012. Two different intervention packages were given to enrolled children for 5 months based on their nutritional status (underweight or normal weight). Underweight children received sachets of supplementary food while both groups received micronutrient supplements (Fig. 1). Neither intervention contained vitamin D supplementation.
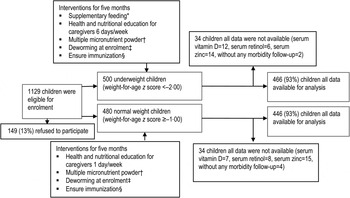
Fig. 1. Study profile. * Only underweight children received supplementary feeding. Each sachet of supplementary food contained 20 g roasted rice powder, 10 g roasted lentil powder, 5 g molasses and 5 ml vegetable oil providing ~150 kcal. Severely underweight (WAZ <–3) and moderately underweight children received three packets and two packets, respectively, 6 days/week for 5 months or until graduation by achieving WAZ – 1. † Both underweight and normal-weight children received multiple micronutrient powder for 2 months (12·5 mg elemental iron, 5 mg elemental zinc, 300 lg vitamin A, 150 lg folic acid and 50 mg vitamin C). ‡ Deworming at enrolment (200 mg albendazole syrup was given orally as a single dose to all children aged >1 year. For children aged <1 year, 10 mg/kg pyrantel pamoate was given as a single dose.) § Immunization covers bacillus Calmette Guérin; diphtheria, pertussis and tetanus; oral polio vaccine; measles; hepatitis B; and Hib vaccines.
Data collection and morbidity surveillance
A longitudinal study design was used to follow both normal-weight and underweight children for a period of 5 months. At the time of enrolment we collected information on socio-demographic factors and household economic conditions using a standard DHS household questionnaire. Trained health fieldworkers collected information regarding common infectious disease illnesses including sign and symptoms of ARI through home visits every third or fourth day for 5 months, using previously validated standardized, morbidity questionnaires. During home visits, caregivers were asked about signs and symptoms of any illness experienced by the child since the last visit. The fieldworkers also measured the respiratory rate of any child suffering from cough with fever or any sign of respiratory distress. Children with signs of cough and respiratory distress, such as difficulty or rapid breathing and chest in-drawing, were referred to the study physicians for evaluation of ALRI and treatment. If any study participants were absent from the study site for >7 days, during the next available visit fieldworkers would then collect morbidity information for the last 7 days only. Any children absent from the household or study site for >60 consecutive days were excluded from the study.
Assessment of micronutrient status
The determination of serum vitamin D and other micronutrients in each child was conducted at enrolment using a 5 ml blood sample collected into trace element-free tubes. Processing of the collected samples and micronutrient assays were performed at the nutritional biochemistry laboratory of icddr,b. Serum vitamin D (25-hydroxy vitamin D) was measured by using IDS 25-hydroxy vitamin D EIA kit [Reference Adams and Hewison24] (IDS Ltd, UK). Serum retinol was measured by the HPLC method described elsewhere [Reference Driskell25]. Serum/plasma zinc concentration was measured with air-acetylene flame atomic absorption spectrophotometer at 213·9 nm following dilution of the sample 12 times with deionized water. The results for serum retinol and zinc status were adjusted for subclinical infections with C-reactive protein and α 1 acid glycoprotein [Reference Thurnham26, Reference Mburu27].
Measurements
We used standard case definitions to identify URI and ALRI from collected information. For the purpose of this study a URI was defined as the ‘presence of cough in absence of World Health Organization-defined clinical signs of pneumonia and severe pneumonia’, while an ALRI was defined as the ‘presence of cough and/or respiratory difficulty plus rapid respiratory rate at the time of household visits (cut-off for age: ⩾50 breaths per minute in children aged 2–11 months and ⩾40 breaths per minute in children aged 12 months to 2 years)’ [28]. If any child was diagnosed with ALRI during episodes of URI, then all days reported with cough were considered as an ALRI episode.
Demographic individual-level variables included age group (6–11, 12–17, 18–24 months) and maternal education (illiterate, 1–5 years and >5 years of institutional education). A household wealth index was constructed using socioeconomic variables recorded in the database using principal component analysis as described in the DHS [Reference Rutstein and Kiersten29]. Four seasons – summer (May–July), autumn (August–October), winter (November–January) and spring (February–April) – were created using the date of blood sample collection. Children's vitamin D status was then categorized into deficient (<50 nmol/l), insufficient (⩾50 and <75 nmol/l) and sufficient (⩾75 nmol/l) according to recommendations of the US Endocrine Society guideline [Reference Holick30]. Serum retinol status was categorized as moderate to severe deficiency (<0·7 μmol/l) and mild deficiency to normal status (⩾0·7 μmol/l) [31]. Serum zinc insufficiency was defined as serum zinc <9·9 μmol/l while sufficient status was defined as ⩾9·9 μmol/l [Reference Brown32].
Complete data was available from 466 underweight and 446 normal-weight children for the analysis (Fig. 1). Study physicians successfully collected blood samples from all the children at the time of enrolment. Assay results for serum vitamin D, retinol and zinc in 32 underweight and 34 normal-weight children were not completed due to sample haemolysis, coagulation or insufficient serum available from collected blood samples to perform the assays. Additionally, parent/caregivers of six participants (four in the underweight group and two in the normal-weight group) did not agree to provide morbidity surveillance data after enrolment in the study and were therefore excluded from the study.
Statistical analyses
The primary outcomes in all analyses were URI and ALRI rates in children during the 5 months of active surveillance period. URI and ALRI rates were calculated as the number of days with URI or ALRI by the total number of days that a child was observed. URI and ALRI rates were then annualized as rate per child per year. In all the analyses, the main exposure of interest was serum vitamin D status at the time of the enrolment. The rates per child year of URI and ALRI were estimated as per age group, sex, maternal and socioeconomic characteristic and serum vitamin D, retinol and zinc status of children. We built generalized estimating equation (GEE) models with a Poisson distribution to estimate incidence rate ratios (IRRs) and 95% confidence intervals for URI and ALRI incidence; an exchangeable correlation structure was specified to account for within-child and within-family correlations of outcome measures. For the multivariable GEE modelling, potential confounders included measures of socio-demographic status and a micronutrient status indicator, which were physiologically deemed important factors or reported risk indicators in the published literature [Reference Zaman6, Reference Gurley33]. P < 0·05 was considered to be a statistically significant association for all the analyses. We estimated adjusted IRRs of URI and ALRI from multivariable models. We constructed four separate models to evaluate the association of micronutrient status and ARI incidence. Model 1 (age, sex, vitamin D) and model 2 (age, sex, vitamin D, retinol, zinc) were constructed to evaluate biological factors of children. Model 3 further adjusted for maternal education and household wealth index. Model 4 comprised all variables in model 3 and was additionally adjusted for more distal environmental factors (season of serum D measurement). All analyses were performed in Stata software v. 13.0 (StataCorp., USA).
Ethical disclosure
The data used in the analysis were collected as part of the MAL-ED Network studies, which have been designed to understand the complex inter-relationship between enteric infections and malnutrition. The MAL-ED study (proposal no. 2008-020) was approved by the Research Review Committee and the Ethical Review Committee of icddr,b in 2008. The caregivers of eligible children provided informed written consent before collection of data and provision of all biological samples at the time of enrolment. The Ethical Review Committee was satisfied with the written participation, and maintenance of all rights to participate in the study. Caregivers could withdraw the child from this study at any time without the loss of treatment.
RESULTS
The status of serum vitamin D, retinol and zinc in underweight and normal-weight children are presented in Supplementary Table S1. In underweight children, 23·4% were classified as vitamin D sufficient while 41·8% and 34·8% were insufficient and deficient, respectively. Around 40·6% underweight children had moderate to severe serum retinol deficiency while 20·0% had zinc insufficiency. Only 15·0% of normal-weight children were vitamin D sufficient at the time of enrolment while 39·5% and 45·5 had vitamin D insufficient and deficient status, respectively. A total of 34·1% normal-weight children had moderate to severe serum retinol deficiency while 16·1% had serum zinc insufficiency.
Association of URIs and ALRIs with vitamin D status in underweight children
Underweight children contributed 62 988 total days of observation and experienced 69·1 days/child-year of URI (Table 1). Of the underweight children, those who were vitamin D deficient (P < 0·001) and insufficient (P < 0·05) experienced significantly fewer days of URI than those who were vitamin D sufficient. Younger (6–11 months) children experienced more days of URI than older children [12–17 months (P < 0·05), and 18–24 months (P < 0·001)].
Table 1. Upper respiratory tract infection (URI) and acute lower respiratory tract infection (ALRI) incidence according to socio-demographic characteristics and micronutrient status (serum vitamin D, retinol and zinc) in underweight children aged 6–24 months †
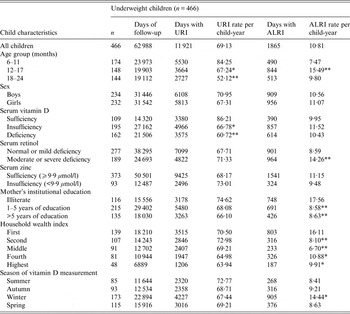
† For ordinal predictors, the categories of the predictor were introduced as continuous into the generalized estimating equation model with a Poisson distribution and P value was a test for linear trend. For dichotomous predictor, P value is from the Wald test with robust standard errors in the generalized estimating equation model.
*P < 0·05, **P < 0·001.
Underweight children experienced 10·8 days/child-year of ALRI. There was no significant association between vitamin D status and ALRI in underweight children. Children aged 12–17 months (P < 0·001) had significantly higher rates of ALRI than the younger group (6–11 months). Underweight children whose mothers had 1–5 years and >5 years of institutional education were found to have significantly lower ALRI rates than children of illiterate mothers (P < 0·001). Household wealth index was inversely associated with the number of days with ALRI in underweight children [fourth and highest (P < 0·05) and second and middle (P < 0·001)]. Moderate to severe serum retinol-deficient children (P < 0·001) experienced more days of ALRI than children with mild to normal retinol status (Table 1).
In underweight children, there was 20% lower risk of URI in vitamin D-insufficient children compared to vitamin D-sufficient children; this result was consistent in all models tested (Table 2). Similarly, there was 25–27% lower risk of URI in vitamin D-deficient underweight children compared to vitamin D-sufficient children. After adjustment for confounders older, underweight children (18–24 months) had 33% lower risk of URI than younger children.
Table 2. Incidence rate ratios and 95% confidence intervals of upper respiratory tract infection and acute lower respiratory tract infection in underweight children aged 6–24 months

Model 1: Adjusted for age and sex.
Model 2: Model 1 + adjusted for serum retinol and zinc.
Model 3: Model 2 + adjusted for maternal education and household wealth index.
Model 4: Model 3 + adjusted for season of vitamin D measurement.
*P < 0·05, ** P < 0·001.
No significant differences were found for ALRI risk in underweight children who differed in vitamin D status (Table 2). After adjustment for confounders in the final model, underweight children, and older children (12–17 months) had 1·8 times higher risk of ALRI than younger children (6–11 months). Similarly, those with severe to moderate serum retinol deficiency had 1·8 times higher risk of ALRI compared to those with mild to normal retinol status. Underweight children of educated mothers (1–5 years and >5 years of institutional education) had 43–38% lower risk of ALRI than underweight children whose mothers were illiterate. Moreover, underweight children from the low-middle and middle stratum of the household wealth index had 52% lower risk of ALRI compared to underweight children from the lowest stratum.
Association of URIs and ALRIs with vitamin D status in normal-weight children
Normal-weight children contributed 62 133 total days of observation and had 78·4 days/child-year of URI (Table 3). Older (12–17 months and 18–24 months) children had fewer days of URI than younger children (6–11 months) (P < 0·05). Children from the highest stratum of the household wealth index had significantly fewer days with URI than children of the lowest stratum (P < 0·05). In normal-weight children, those who were serum zinc insufficient had a greater number of days of URI than those who were zinc sufficient (P = 0·05).
Table 3. Upper respiratory tract infection (URI) and acute lower respiratory tract infection (ALRI) incidence according to socio-demographic characteristics and micronutrient status (serum vitamin D, retinol and zinc) in normal-weight children aged 6–24 months †
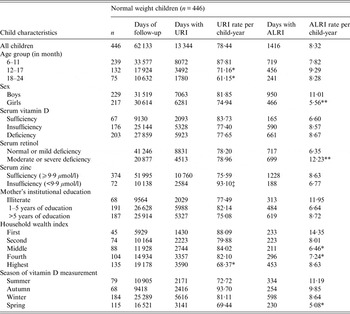
† For ordinal predictors, the categories of the predictor were introduced as continuous into the generalized estimating equation model with a Poisson distribution and P value was a test for linear trend. For dichotomous predictor, P value is from the Wald test with robust standard errors in the generalized estimating equation model.
‡ P = 0·05, *P < 0·05, **P < 0·001.
Normal-weight children had 8·3 days/child-year of ALRI. Normal-weight girls had fewer days with ALRI than boys (P < 0·001). In normal-weight children, measurement of vitamin D status during spring was associated with reduced ALRI compared to summer (P < 0·05). Normal-weight children from middle and high-middle stratums of the household wealth index had fewer days with ALRI than normal-weight children from the lowest stratum of the index (P < 0·05). Moreover, in normal-weight children, those with moderate to severe serum retinol deficiency had more days with ALRI than those with mild to normal serum retinol status (P < 0·001) (Table 3).
Vitamin D status in normal-weight children was not associated with URI across all the models. There was a 20% and 31% reduced risk of URI in children aged 12–17 months and 18–24 months, respectively, compared to younger children (6–11 months). Normal-weight children with insufficient zinc status had 1·2 times greater risk of URI compared to normal-weight children with sufficient serum zinc status. There was 26% lower risk of URI in normal-weight children from the highest wealth stratum compared to normal-weight children from the lowest wealth stratum. Of normal-weight children, measurement of vitamin D status during autumn indicated a 1·3 times greater risk of URI compared to measurement of vitamin D status during summer (Table 4).
Table 4. Incidence rate ratios 95% confidence intervals of upper respiratory tract infection and acute lower respiratory tract infection in normal-weight children aged 6–24 months
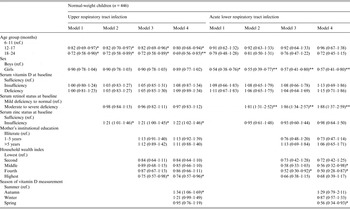
Model 1: Adjusted for age and sex.
Model 2: Model 1 + adjusted for serum retinol and zinc.
Model 3: Model 2 + adjusted for maternal education and household wealth index.
Model 4: Model 3 + adjusted for season of vitamin D measurement.
*P < 0·05, ** P < 0·001.
No significant differences in the IRRs of ALRI were found between normal-weight children who differed in vitamin D status in any of the models. After adjustment of confounders in the final model, girls had 43% lower risk of ALRI than boys. There were 1·9 times greater risk of ALRI in normal-weight children with severe to moderate serum retinol deficiency compared to normal-weight children with mild deficiency and normal serum retinol status. Normal-weight children from the low-middle and high-middle stratum of the household wealth index had 44% and 50% lower risk of ALRI, respectively, than normal-weight children from the lowest stratum. Measurement of vitamin D status during spring had 44% lower risk of ALRI in normal-weight children compared to measurement of vitamin D status during summer (Table 4).
DISCUSSION
The results of our study provide new insight into the role of vitamin D status alone, as well as in combination with other micronutrient status and socioeconomic indicators, in the incidence of ARI in underweight and normal-weight children aged 6–24 months. The findings of the present study demonstrate that underweight children with vitamin D deficiency or insufficiency had reduced risk of URI compared to underweight children with sufficient vitamin D status. This is the first reported finding indicating that vitamin D insufficiency or deficiency in underweight children might protect children aged 6–24 months against URIs. Results of RCTs in children examining the effect of vitamin D supplementation on respiratory tract infection have not been consistent [Reference Manaseki-Holland15, Reference Manaseki-Holland34]. One study found no association in the incidence of ARI with vitamin D supplementation but did report that supplementation was associated with an increase in repeated episodes [Reference Manaseki-Holland15]. A recent RCT in older adults also reported increased risk and duration of URI with intermittent bolus dose supplementation of vitamin D [Reference Martineau35]. These results support our findings in underweight children. Available evidence indicates that vitamin D upregulates the innate immune response but inhibits any overzealous responsivity of the stronger adaptive immune response to offending infections and antigens [Reference Schwalfenberg36]. Continuous and overwhelming infections experienced by children included in our study could partially explain our findings that they suffered 69–78 days of URI annually and live under poor caring practices and socioeconomic status. Furthermore, the role of vitamin D in triggering the immune system in undernourished children is yet to be explored. Additionally, air pollution in urban slums such as the study area is one of the most important factors for higher incidence of ARI in children [Reference Gurley33, Reference Romieu37]; unfortunately we were not able to account for this in our analyses. Moreover, additional food supplementation in underweight children could also be contributing such findings.
We found that the risk of URI in children was significantly lower in both underweight and normal-weight older children (12–24 months) in all multivariable models; this finding contrasts with that from a previous study carried out in rural Bangladesh [Reference Zaman6]. Our study was based in an urban slum setting with participants living in a more polluted environment. A study recently conducted in the same field site reported exposure to high indoor particulate matter (PM 2·5) associated with childhood respiratory tract infections [Reference Gurley33]. The high burden of polluted environment with possible immature immunity at young age is likely to contribute to young children (6–11 months) being more vulnerable to URI than older children.
In a recent review, it was reported that in preschool children, those receiving vitamin A supplementation had no significant difference in incidence of pneumonia compared to those in a placebo group [Reference Mathew38]. However, in our study we found that both normal-weight and underweight children with moderate to severe serum retinol deficiency had a higher risk of ALRI. These findings could be explained by the known role of serum retinol in enhancing the regeneration and integrity of respiratory and gastrointestinal epithelia and in modulating the immune function [Reference Thorne-Lyman and Fawzi39].
We did not find any difference in risk of ALRI incidence in children with insufficient zinc status compared to children with adequate zinc status; this finding is supported by a recent review in children aged 6 months to 12 years [Reference Mayo-Wilson40]. However, normal-weight children classified as zinc insufficient were found to have a greater risk of URI than zinc-sufficient children. This finding is in line with a study on adults, reporting a reduction in symptoms of the common cold in adults receiving a zinc supplement [Reference Veverka41].
Underweight children aged 12–17 months had significant risk of ALRI compared to the 6–11 months age group. In developing countries, WAZ score is stable during first year of life but is followed by a period of progressive and slow faltering until children reach the age of 5 years [Reference Victora42]. This critical period of progressive faltering may lead to a reduced immune response and so increase the risk of ALRI [Reference Rodriguez, Cervantes and Ortiz21]. Female normal-weight children had significantly less risk of ALRI than boys after adjusting for all other potential confounders. A similar finding for URI in children aged 5–11 years was also reported in a study conducted in rural Bangladesh [Reference Torres43].
A study done in the same site did not find any association of maternal elementary education and the risk of ALRI [Reference Gurley33]. However, in our study, underweight children of mothers with institutional education showed a lower risk of ALRI than children of illiterate mothers. Possible explanations for this finding are that educated mothers have better childcare practices [Reference Kulwa, Kinabo and Modest44], thus, preventing children from progressing to more severe forms of the disease. In both underweight and normal-weight children, those from higher socioeconomic households had a lower risk of ALRI than those from lower socioeconomic households. These findings are supported by two small studies conducted in urban slums and rural settings in Bangladesh [Reference Rahman and Rahman45, Reference Rahman and Shahidullah46].
The strength of our study is that we documented the respiratory morbidity events through twice-weekly home visits which allowed us more adequate disease identification by minimizing reporting bias and allowing for adequate documentation of the duration of the morbidity events [Reference Lee47]. Provision of clinical care at the study site and the referral of children to specialized hospitals for severe illness may have resulted in better identification of ARI events but may also have promoted over-reporting of illnesses, ultimately resulting in reporting bias by the caregiver. Longitudinal study designs, moderate sample size, and a low rate of loss to follow-up also contributed to the strength of the study. Furthermore, the incidence rate of URIs and ALRIs reported in this study is concordant with other studies conducted in resource-limited settings [Reference Zaman6].
The findings of this study should be interpreted in light of its limitations. First, both groups of children received multiple micronutrient powder supplementations, as recommended by the National Nutrition Program of Bangladesh, which included vitamin A and zinc, but not vitamin D. The role of supplementation with vitamin A and zinc was not considered for incidence of ARI in these analyses due to fewer number of serum collected for micronutrient assays at the end of follow-up, which may confound the findings of our results. Only underweight children received supplementary food and intensive health and nutritional education (Fig. 1) that may have resulted in our findings of lower incidence of URI in underweight children than normal-weight children. However, the severe form of ALRI incidence was found to be higher in underweight children than normal-weight children. Second, the diagnosis of URI in this study relied on the presence of cough but did not consider other signs such as nasal discharge. Similarly, for ALRI events we did not consider central cyanosis or oxygen saturation or X-ray test results or culture for causative organisms for diagnosis of ALRI events, which could strengthen our results. Third, we missed some of the ARI episodes due to absence of the child and caregiver from the household. At least 120 days of morbidity surveillance information was successfully collected from 87·1% and 90·1% underweight and normal-weight children, respectively. Fourth, other micronutrients (e.g. iron status) not considered for the analyses could minimize the relationship found in the current study. Finally, we did not measure the maternal vitamin D status or exposure of sunlight on bare skin, which is the main source of vitamin D in humans. Because we used data from a MAL-ED community-based study with a prospective case-control design and urban setting, results may not be representative of the general population of children in Bangladesh, and specifically those in rural areas.
CONCLUSIONS
Our study demonstrated that serum retinol deficiency has significant association with increased ALRI incidence in both underweight and normal-weight children aged 6–24 months, indicating that efforts to increase the intake of retinol-enriched food or the implementation of supplementation programmes in this population should be encouraged. The mechanisms through which vitamin D can have an effect on the incidence of childhood respiratory tract infections in underweight and normal-weight children still warrants further investigation.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268816001771.
ACKNOWLEDGEMENTS
The Aetiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill and Melinda Gates Foundation, the Foundation for the NIH and the National Institutes of Health, Fogarty International Center. The authors thank all the staff and participants of the MAL-ED Network for their important contributions.
DECLARATION OF INTEREST
None.








