INTRODUCTION
Foodborne diseases cause a serious public health problem worldwide [Reference Guerrant1–Reference Kuchenmüller4]. A recently published study estimated that the yearly global number of non-typhoidal Salmonella gastroenteritis is 93·8 million cases of which 80·3 million are foodborne [Reference Majowicz5]. In Israel about 5000 new cases of shigellosis and 1500 new cases of salmonellosis were reported in 2004 [6]. Diarrhoea is the most common symptom of foodborne diseases [Reference Guerrant1, 7–Reference Guerrant10]. Primarily, the very young, the elderly, and the immunocompromised are affected. The age-specific incidence rates are highest for children aged <3 years, as shown in family studies [Reference Guerrant10].
The burden of foodborne diseases is still unknown and highly underestimated [Reference Hird11–Reference Kubota15]. The incidence rates are usually based on data of laboratory-confirmed cases of diarrhoeal disease. To have a reported case of diarrhoeal disease means that the patient must first seek medical care, a stool specimen must be obtained and submitted to a laboratory for culture, the laboratory must test for and isolate the organism, and the positive culture result must be reported to a health department. If any step in this process does not occur, the illness will not be reported. The burden-of-illness pyramid is a model for understanding foodborne disease reporting. The pyramid demonstrates the chain of events that must occur for an episode of illness in the population to be registered in surveillance [Reference Kuchenmüller4, 16, Reference Scallan17].
The detection of foodborne outbreaks in which the cases of disease are widely spread is dependent on reliable surveillance data. A decrease in stool culturing or reporting would have serious negative consequences for public health surveillance [Reference Guerrant1, Reference Kuchenmüller4, Reference Hennessy18–Reference Herwaldt and Beach23].
In 1996 the U.S. Foodborne Disease Active Surveillance Network (FoodNet) working group conducted a survey in order to understand physician practices regarding the diagnosis of acute diarrhoeal diseases. The study showed that a bacterial stool culture was requested by 44% of physicians for the last patient seen with acute diarrhoea. In that study substantial variability in stool culture request practices was demonstrated among physicians from different geographic areas and specialities [Reference Hennessy24]. A study conducted after a Salmonella enteritidis outbreak in 1994 determined that only 0·3% of the cases associated with this outbreak were culture confirmed. This degree of under-detection is common and demonstrates the low sensitivity of the surveillance system for enteric diseases [Reference Guerrant1]. A study conducted in 1996–2003 by the U.S. FoodNet working group, among individuals with diarrhoea, demonstrated that only 19·5% of these individuals visited a physician for treatment and of these, only 20% were asked by the physician to provide a stool specimen for testing [Reference Jones25].
In order to estimate the burden of diarrhoeal diseases in children and adolescents and to assess the factor of underestimation in Israel, we conducted a nationwide population-based phone and mail survey among paediatricians regarding their knowledge, attitudes and practices. We also analysed a computerized database of a large health maintenance organization (HMO) regarding stool culture and patient visits.
METHODS
Population survey
A population-based telephone survey was conducted during August, September and October 2005. In total 3141 phone numbers were selected randomly from the Israel phone directory. Respondents were phoned on 4 days a week between 16:00 and 21:30 hours. When there was no response, two additional calls were made the same day and four additional calls on two different days. Interviews were conducted in Hebrew, Russian, Arabic or English. We always tried to interview the primary caregiver or, when not available, the first available adult.
Respondents (all aged >18 years) who reported that a child or adolescent in the household had suffered from diarrhoea within 2 weeks prior to the interview were asked about the occurrence of fever >38°C, if there were ⩾3 bowel movements during a 24-h period, the child's capability to attended school (or kindergarten) and the duration of the disability. These respondents were also asked whether the child or adolescent visited a healthcare provider for this illness, if the child took antibiotics for the illness, if the healthcare provider had requested a stool sample, if the child provided a stool sample for diagnostic purposes and whether the child was hospitalized because of the illness. Respondents were also asked about demographic and socioeconomic characteristics. The average salary was defined as the average gross salary published by the Israeli Central Bureau of Statistics.
Diarrhoea was defined as ⩾3 loose stools in a 24-h period [Reference Majowicz26].
The response proportion for this survey was calculated using the response proportion estimation formula provided by the Council of American Survey Research Organization (CASRO) adapted to telephone health surveys [Reference White27].
The study was approved by the Tel Aviv University Helsinki Committee.
Physicians' survey
We conducted a population-based mail survey of paediatricians in Israel to determine the burden-of-illness pyramid for diarrhoeal disease of Israeli children. In addition, we examined the characteristics of both children and paediatricians associated with a stool specimen request. All the Israeli population has medical governmental insurance and receives medical services by HMOs. Visiting a paediatrician and performance of stool culture do not require any co-payment. The study was conducted in collaboration with Maccabi Healthcare Services (MHS). MHS is the second largest HMO (in terms of number of insured members) operating in Israel. Four hundred and forty-four questionnaires were mailed to all of the MHS paediatricians during February and March 2006. The goal was to obtain a 60% response rate.
The two-page questionnaire was accompanied by a letter from the director of primary care of MHS, who encouraged the physicians to complete the questionnaire and explained the objectives of the survey and the intended uses of the data. No financial incentives were offered to respondents.
If no response was received after a month, a telephone call was made in order to verify the mailing address and encourage participation. After the telephone call a new questionnaire was mailed.
The questionnaire included questions concerning the socio-demographic characteristics of the paediatrician's population, the main factors that affect the physician's decision to request a stool culture, the mean number of patients with symptoms of diarrhoea who generally seek care every week, the mean number of patients requested to supply a stool specimen for culture, the percentage of stool cultures actually performed, the percentage of positive cultures, the impact of the percentage of positive cultures on the request for a stool culture and the paediatrician's knowledge regarding the laboratory's ability to detect various enteropathogens.
Medical database
The study was conducted at MHS. A retrospective population-based cohort study of all patients aged <17 years with a diagnosis of diarrhoea from January 2004 to December 2005 was performed. All documented diarrhoeal episodes in the computerized clinical patient's records were included in the study. Diarrhoea episode was defined as the appearance in the patient's record of an ICD-9-CM diagnosis of diarrhoea or diarrhoeal-related diagnosis (ICD-9-CM codes 003-009, 558.9, 787.91). A new diarrhoeal episode was determined by the absence of diarrhoea or related diagnosis in the 14-day period prior to the episode. A stool culture was considered related to a diarrhoeal episode if it was provided after the first visit to the clinic because of diarrhoea and up to ⩽7 days from the last visit.
Variables relating to demographics (age and gender) and microbiological data were extracted from the medical records and laboratory databases.
Statistical analysis
We used data from the three sources mentioned above to assess the factor of underestimation. This factor derives from a chain of events from occurrence of cases of diarrhoea in the general population to reporting. Each source provided data for one or more events. In case of more than one assessment for an event, we used the most reliable one, e.g. the largest sample size.
Data were compiled into three datasets using a database management system (Access, Microsoft Corp., USA). Prevalence proportions and 95% confidence intervals (CI) were calculated. Differences in proportion were assessed by χ2 tests. Differences in means were assessed by t test and Kruskal–Wallis test. Multivariate logistic regression and χ2 automatic interaction detector (CHAID) were used to identify factors influencing performance of stool culture. A P value ⩽0·05 was considered significant. Segmentation and statistical analysis were performed using SPSS software version 14 (SPSS Inc., USA).
We used Monte Carlo simulation in order to estimate the factor of the underestimation of diarrhoeal diseases in Israel. Monte Carlo simulation is based on random sampling from a given distribution. We used beta distribution for the following proportions: visiting a physician, request to provide a stool specimen, providing a stool specimen for culture, positive laboratory cultures and reporting. Mean and 95% confidence interval of each of these proportions were taken from the surveys. If there were more than one assessment for a proportion, we used the one with the largest sample size. We estimated the reporting proportion based on a previous survey [28] and current professional opinion. We made 1000 simulations, of 10 000 scenarios each. In each Monte Carlo scenario, a random sampling is taken from each of the five distributions and all of the samples are multiplied in order to evaluate the chance that a virtual person with diarrhoea will be reported to the Ministry of Health. The final parameter of each simulation is the mean factor of the underestimation. Monte Carlo simulation was performed using Matlab (R2008a, The MathWorks Inc., USA), and statistical analysis was performed employing SPSS software version 14 (SPSS Inc.).
RESULTS
Population survey
Overall, 3141 interview attempts were made, resulting in 1769 households agreeing to participate. The response rate among those who were approached was 74% (1769/2395). After excluding 1089 households without a child aged <17 years, 680 complete interviews were included for analysis (Fig. 1). The 680 households comprised 1492 participants (children and adolescents aged <17 years).
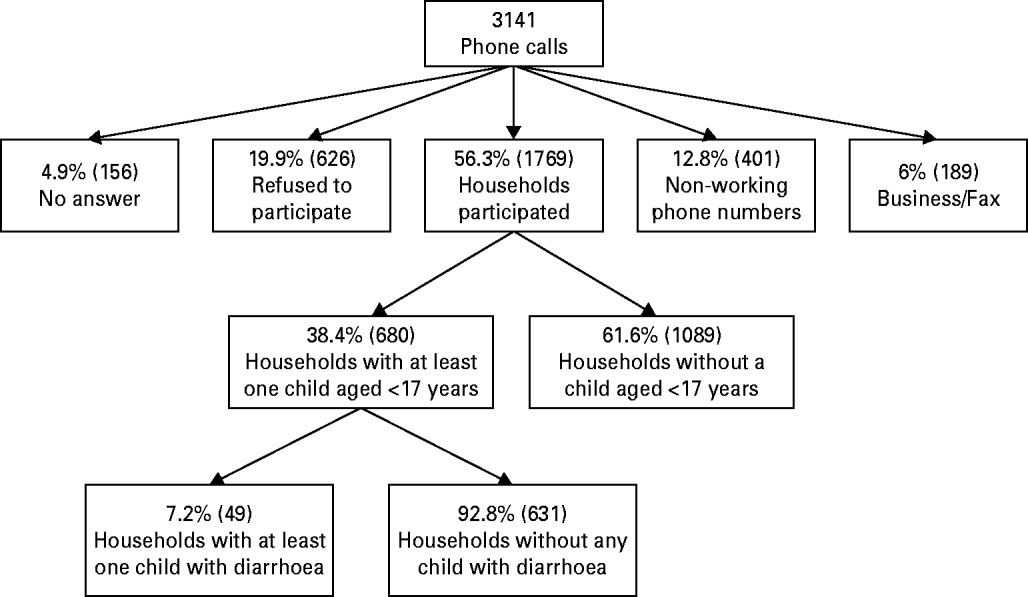
Fig. 1. Flow chart of household selection process.
Of the 1492 study participants aged <17 years, 85 had diarrhoea in the 2-week period preceding the interview (prevalence 5·7%, 95% CI 4·6–7). This represents a rate of 1·49 episodes of diarrhoea per year for each child and adolescent aged <17 years.
Statistically significant differences were detected in the prevalence proportions of diarrhoea according to age and region of residence. The prevalence of diarrhoea was highest (9·1%) in children aged <5 years and lower (4·3%) above this age (P=0·007). The prevalence of diarrhoea was highest (10·9%) in children living in Central Israel and lowest in those living in Northern Israel (3·5%) (P=0·017).
The prevalence of diarrhoea did not vary significantly among ethnic groups (P=0·221), according to mother's education (P=0·865), residence (P=0·876) and their HMO (P=0·861).
Of participants with diarrhoea, 32·9% (95% CI 23·9–43·5) reported having fever >38°C and 11·8% (95% CI 6·5–20·3) took antibiotics. None of the patients was hospitalized because of diarrhoea but 55·3% (95% CI 44·7–65·4) were unable to attend school, kindergarten or summer camp. Of these, 80% stayed at home for 1–4 days, 15·6% for 5–8 days and 4·4% for ⩾9 days. In all cases, an adult had to take care of children who stayed at home. In most cases (69·6%), the mother was the caregiver, then the father (14·3%) and in fewer cases (5·4%) the grandfather/grandmother, brother or nanny.
For individuals suffering from diarrhoea, the reported proportion that visited a physician was 38·8% (95% CI 29·2–49·5). Of these only 21·2% (95% CI 10·7–37·8) were requested to provide a stool specimen. All the individuals who were requested to provide a stool specimen complied with the request.
The proportion visiting a physician was highest (50%) for children aged <5 years, 38% for children aged between 5 and 8 years, and lowest (20%) for those aged ⩾9 years. Antibiotics were prescribed to 27·3% (95% CI 15·1–44·2) of the patients visiting a physician because of diarrhoea.
Physicians' survey on practices regarding diarrhoeal diseases
Forty-eight percent (214/444) of the questionnaires were returned. Seventy-five percent (159/211) of the physicians interviewed defined the population that they served as of middle income and only 15·6% (32/211) and 9·4% (20/211) took care of patients of low and high income, respectively. Ninety-three percent (199/213) of physicians reported that the majority of patients in the population they served were of Jewish origin. Forty-seven percent (100/213), 12·1% (26/213) and 40·7% (87/213) defined the population they served as non-religious, religious and mixed, respectively.
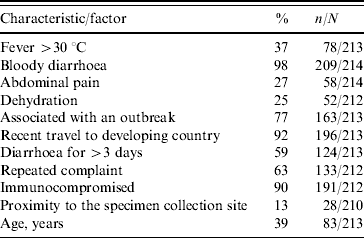
Reported bloody diarrhoea, a condition of immunosuppression, and a report on recent travel to a developing country were the factors which had the strongest impact on the physicians' decision to order a stool culture (Table 1). When asked to choose, 72% of the physicians ranked ‘bloody diarrhoea’ as the complaint which influenced them the most regarding the ordering of a stool culture. For 10% of physicians, persistence of diarrhoea for >3 days, was the most important factor which determined their decision to order a stool culture. The identification proportion of enteropathogens in stool samples collected from patients with diarrhoea is low. This is due to a limited number of pathogens that the laboratory attempts to detect routinely as well as the relatively low sensitivity proportion even of the classical stool culture, as well as faulty collection procedures. Nevertheless, only 26·2% (53/202) of physicians took this factor into consideration when they considered requesting a stool culture. It appears that paediatricians assume that the laboratory routinely tests for more pathogens than they actually do. Most physicians assume that the laboratory always tests for Salmonella and Shigella (97·7%, 99·1%, respectively) which is in fact correct. However, they also assume that the laboratory tests for additional pathogens as well (Campylobacter 62·0%, E. coli O157 38·0%, Yersinia 2·8%, Vibrio 0·5%, other pathogens 6·6%), while in practice this is performed only by special request.
Table 1. Factors considered by paediatricians as determinants for stool culture requests
Physicians reported requesting 24% (s.d.=11·6%) of the patients to supply a stool specimen. They also reported 75% compliance with their request.
Medical database
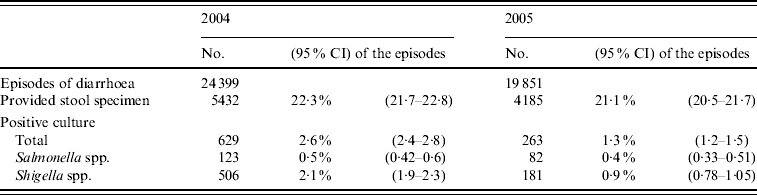
In 2004–2005, 44 250 episodes of diarrhoea were diagnosed. Stool cultures were performed in 21·7% (9617, 95% CI 21·4–22·1) of these. Shigella and Salmonella spp. were identified in 1·5% and 0·5% of the diarrhoeal episodes, respectively (Table 2). The isolation proportions per culture obtained were 7·1% for Shigella and 2·1% for Salmonella. Although the proportion of stool cultures performed was highest during the first 3 years of life (22·2–28·1%), the isolation proportion was low (3·1–13·5%) (Fig. 2). In 2004, the number of episodes and the isolation proportion were higher than for 2005 (24 399 vs. 19 851, 11·6% vs. 6·3%, respectively).
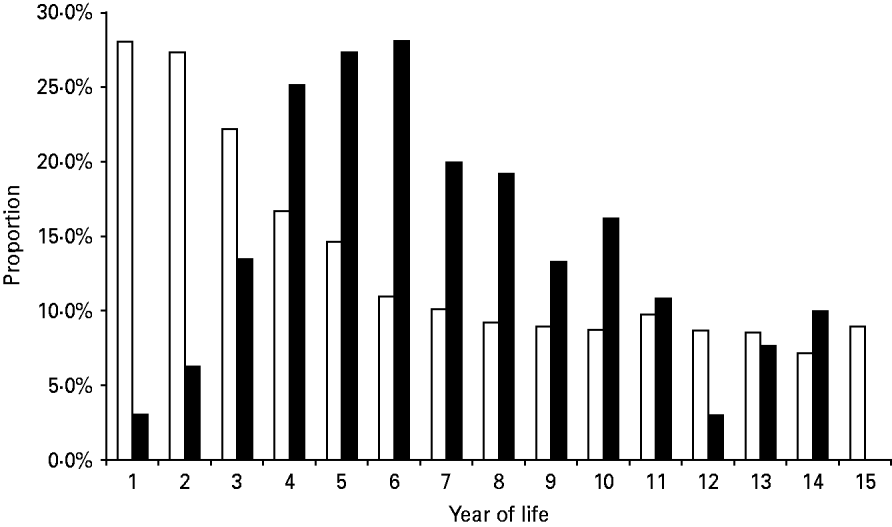
Fig. 2. Proportions of stool cultures performed per diarrhoeal episode (□) and Shigella and Salmonella positivity per culture (▪), by age.
Table 2. Episodes of diarrhoea, stool cultures performed and isolates from Maccabi Healthcare Services database
A CHAID analysis revealed that age was the most important factor for performing stool cultures with six different subgroups [age⩽0·6, performance proportion (perp)=30·6%; 0·6<age⩽1·9, perp=27·0%; 1·9<age⩽2·4, perp=24·8%; 2·4<age⩽3·2, perp=19·8%; 3·2<age⩽5·0, perp=15·3%; age>5·0, perp=9·5%; P<0·001]. The CHAID analysis also demonstrated that there were significant differences in performing stool culture between 2004 and 2005 only in patients in the 3rd and 4th age groups (1·9<age⩽2·4: perp2004=26·4%, perp2005=22·7%, P=0·005; 2·4<age⩽3·2: perp2004=21·3%, perp2005=17·7%, P=0·003). In 2004, there were more diarrhoeal episodes and higher isolation proportions compared to 2005. This difference was associated with a higher proportion of stool cultures performed in 2004 in patients aged 1·9–3·2 years.
Based on the two surveys and the medical database we were able to determine the burden-of-illness pyramid which shows the flow of information from cases of diarrhoea occurring in the general population to their reporting to the Department of Epidemiology at the Ministry of Health (Fig. 3).
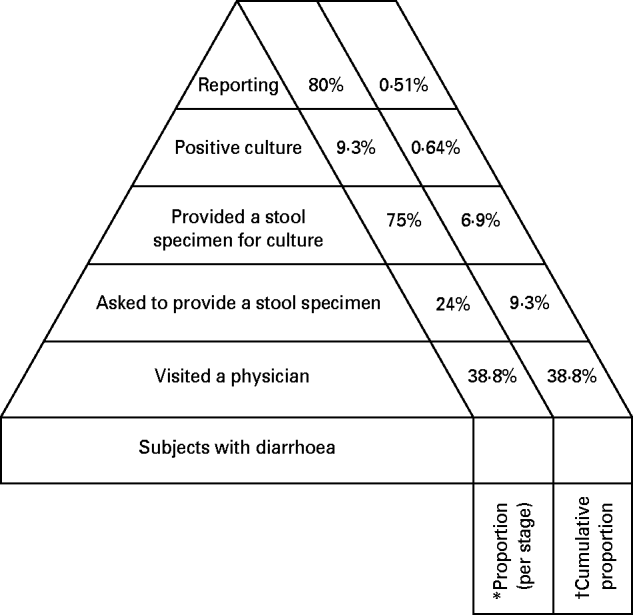
Fig. 3. Proportion of each stage from visit to physician to reporting (the burden-of-illness pyramid). * Percentage of the category/stage below. † Percentage of individuals with diarrhoeal disease in the general population.
DISCUSSION
We performed two surveys and used one database to establish the burden-of-illness pyramid and to evaluate the factor of underestimation of diarrhoeal diseases in children in Israel.
The prevalence of diarrhoea in this study was 5·7% in the 2 weeks prior to interview according to the population-based telephone survey. This proportion is substantially higher than that in the FoodNet population surveys (5·1% in the previous month) [Reference Jones25]. This difference can be explained by the different definition used in our study (diarrhoea) and in the FoodNet surveys (acute diarrhoeal illness) [Reference Jones25, Reference Majowicz26]. In addition, our study referred to a specific age group with relatively high prevalence while the estimate published by FoodNet referred to all ages [Reference Jones25, Reference Scallan29]. Our results are consistent with age-matched estimates from Australia (age<5, 8·2%; 5–14, 4·8%), Canada (age<5, 11·7%; 5–14, 5·2%), Ireland (age<5, 7·6%; 5–14, 4·7%) and USA (age<5, 11·2%; 5–14, 8·3%) [Reference Scallan29]. Differences that still persist can be explained by the various predominant enteropathogens and incidence rates in different countries, such as Campylobacter infections in the USA and Australia [Reference Vally30]. The proportion seeking a physician in our study (39%), is much higher than the proportion (20%) reported by FoodNet for 1996–2003 [Reference Jones25], possibly being associated with a higher utilization of healthcare services in Israel which is partially attributable to no co-payments. We assume that the proportion seeking care is almost the same during the whole year. Despite the known seasonality of various diarrhoeal diseases and enteropathogens we are not aware of parallel changes in either medical services or medical practices. In addition, the climate in Israel does not influence the accessibility to medical services throughout the year.
A severe symptom of bloody diarrhoea, recent travel to an endemic area (developing country) and an immunosuppressed condition had the strongest impact on the physician's decision to request a stool culture.
FoodNet reported that in USA the most important factors associated with submission of stool specimens were bloody diarrhoea and diarrhoea duration of ⩾3 days [Reference Hennessy18]. From the medical database, patient's age and a higher incidence of diarrhoeal diseases emerged as important determinants of requests for stool cultures.
The reported proportion of stool cultures performed was similar for all three data sources (population survey 21·2%, physicians' survey 17·8%, medical database 21·7%). These proportions are close to the figure (18%) reported by FoodNet for 1996–2003 [Reference Hedberg and Hirschhorn19].
The medical database of MHS also showed that from 1000 patients seeking care about five will be identified by the laboratories as suffering from salmonellosis and 16 as suffering from shigellosis. The significantly higher Shigella isolation proportion in 2004 was associated with the epidemic circulation of S. sonnei in the community during that year compared to 2005. All the Israeli population has government-funded insurance and MHS is the second largest HMO. We assume that the data obtained in this study on MHS paediatricians' knowledge, attitudes and practices related to patient visits with diarrhoeal diseases can be extended to all the paediatricians in Israel.
In 1996 only about 65% of the enteropathogens isolated at clinical microbiological laboratories were reported to the Department of Epidemiology of the Ministry of Health [28]. It is currently estimated, due largely to the improvement in computerization of laboratories, that the reporting proportion is between 70% and 90% (mean 80%).
In the British Columbia region, for every case of infectious gastrointestinal illness reported to the province, a mean of 347 (95% credible interval (CrI) 181–611) community cases occurred [Reference MacDougall13]. In Australia, the multiplier for salmonellosis was estimated at 7 (95% CrI 4–16), for campylobacteriosis at 10 (95% CrI 7–22), and for Shiga toxin-producing E. coli infections at 8 (95% CrI 3–75) [Reference Hall14].
Using Monte Carlo simulation we estimated that one reported isolate of Shigella or Salmonella represents 152 (95% CrI 45-383) diarrhoeal episodes of all aetiologies in individuals aged <17 years in the community. As mentioned above, we used data from the three sources to assess the factor of underestimation. We could not evaluate this factor separately for Shigella and Salmonella because we had no information from a single database on individuals followed up through all the chain of events, namely from occurrence of cases of diarrhoea in the general population to reporting. A similar approach is presented in a Canadian study [Reference MacDougall13] on underestimation of diarrhoeal disease burden.
We conclude that the periodical reports of the Department of Epidemiology in the Ministry of Health represent <1% of the diarrhoeal episodes occurring each year in Israel in children and adolescents aged <17 years.
Based on this estimation and the number of episodes of shigellosis and salmonellosis published by the Department of Epidemiology of the Ministry of Health for 2004–2005, we assume that in children and adolescents aged <17 years about 879 000 (95% CrI 260 000-2 214 000) episodes of diarrhoea occurred in Israel in 2004 and about 414 000 (95% CrI 122 000-1044 000) episodes occurred in 2005.
These figures may constitute an underestimation of the real burden of diarrhoeal diseases in the population. The population survey was performed between August and October. While including summer months with relatively high incidence of bacterial diarrhoeal diseases it did not cover winter months with higher incidence of diarrhoeal diseases of viral aetiology. However, we used Monte Carlo simulation to control for incidence changes throughout the year. The proportion seeking a physician in our study (39%) is high and could be influenced by recall bias towards an overrepresentation in our population survey of individuals with more severe diarrhoea, i.e. those with a greater likelihood of visiting a physician. Despite the potential underestimation, these estimates of the burden of diarrhoeal diseases in Israel may serve as basis to develop diarrhoeal prevention programmes.
ACKNOWLEDGEMENTS
The authors thank Search for Common Ground and the Nuclear Threat Initiative non-governmental organizations for supporting the study as part of the Middle East Consortium on Infectious Disease Surveillance activities.
DECLARATION OF INTEREST
None.







