Streptococcus pneumoniae is a common cause of serious infections such as pneumonia, bacteraemia and meningitis. Fatalities often occur and are associated with failing antimicrobial therapy due to antimicrobial resistance [Reference Van Kerkhoven1]. A European network of national surveillance systems for antimicrobial resistance (EARSS) was established in 1998 and is now called the European Antimicrobial Resistance Surveillance Network (EARS-Net). EARS-Net gathers data on the susceptibility testing results of S. pneumoniae and other bacteria isolated from blood and cerebrospinal fluid (CSF) over time and regions. Full details on the aims and protocol of EARS-Net are available online (http://ecdc.europa.eu).
In the clinical laboratory, resistance of S. pneumoniae to penicillin is assessed by screening with the oxacillin (1 μg) disk diffusion test, and determination of the minimum inhibitory concentration (MIC) in cases where the screening test indicates decreased susceptibility. The result is a classification into ‘susceptible’, ‘intermediate’ and ‘resistant’ categories and the cut-off values between these categories are termed clinical breakpoints. These are defined by several national and international organizations, among which some of the most widely used are the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Between 2000 and 2012, the Belgian data for EARS-Net S. pneumoniae antimicrobial susceptibility were constructed each year using the most recent CLSI breakpoints. In January 2008 CLSI changed their S. pneumoniae breakpoints and these were implemented in Belgium from 2009 onwards. After a relatively stable period, EARS-Net reported a significant decreasing trend (from 9·4% to <1%) in Belgian S. pneumoniae isolates non-susceptible to penicillin for the years 2007–2010 [2].
In this paper we aim to quantify the influence of the change in breakpoints on the sudden drop in the proportion of penicillin non-susceptible isolates documented by EARS-Net, and to determine whether this decrease was statistically significant if the change of breakpoints is taken into account. Further, we investigated the effect a change towards EUCAST breakpoints might have – as progressively scheduled in Belgium from 2012 onwards – on the results of the S. pneumoniae surveillance in EARS-Net.
All Belgian clinical laboratories are requested to send all S. pneumoniae isolates to the National Reference Laboratory (NRL), located at the University Hospital, Leuven. The NRL performs capsular serotyping and confirmation of antimicrobial susceptibility test results on all isolates, and generates feedback to the participating laboratories. In order to be legally recognized, all Belgian clinical laboratories must participate in the external quality assessment (EQA) organized by the Belgian Scientific Institute of Public Health (WIV-ISP); 87% of eligible laboratories also participate in the voluntary EARS-Net EQA [2].
The WIV-ISP receives all S. pneumoniae antimicrobial susceptibility data from the NRL and selects only blood and CSF isolates, according to EARS-Net criteria [2]. After verification the data are sent to the European Centre for Disease Control and Prevention (ECDC), the current coordinator of EARS-Net. In the present study isolates with sample dates between 1 January 2003 and 31 December 2010 were included.
In accordance with CLSI guidelines the NRL first screens all S. pneumoniae isolates for susceptibility to penicillin by the Kirby–Bauer disk diffusion method with a 1 μg oxacillin disk. Isolates with an inhibition zone diameter ⩾20 mm are considered susceptible to penicillin. For all isolates with inhibition zones <20 mm the MIC is determined by E-test on Mueller–Hinton agar with 5% horse blood. S. pneumoniae ATCC 49619 with intermediate penicillin resistance and a local isolate of known penicillin susceptibility are used as internal quality controls [Reference Marshall3, 4].
The old (pre-2008) and new (post-January 2008) CLSI breakpoints and the recently introduced EUCAST breakpoints are described in Table 1. Because the Belgian EARS-Net data showed that 96% of the blood isolates were from hospitalized patients, only the intravenous (i.v.) breakpoints were applied (no oral therapy is advised in these cases or in meningitis). It should be noted that the new CLSI breakpoints are much higher for non-meningitis isolates, but remained the same for meningitis isolates. The new breakpoints were then applied to all isolates that were non-susceptible in the disk screening test as MIC data were available for all of them. Where both blood and CSF isolates from the same patient were available, priority was given to CSF isolates.
Table 1. CLSI and EUCAST MIC breakpoints* for S. pneumoniae susceptibility to penicillin
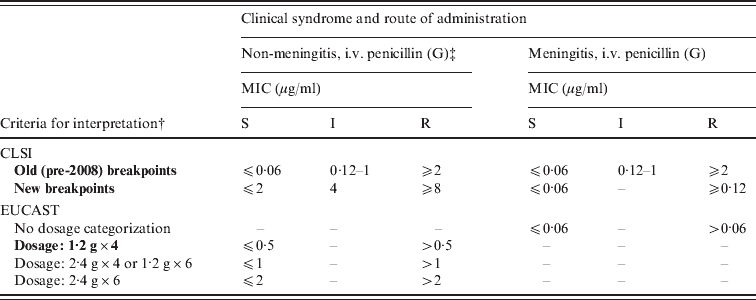
CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; i.v., intravenous; MIC, minimum inhibitory concentration; S, susceptible; I, intermediate; R, resistant.
* Adapted from CLSI and EUCAST Clinical Breakpoint Table v. 2.0, valid from 1 January 2012.
† Breakpoints in bold are those applied in the present study; for EUCAST 1·2 g × 4 was chosen because this most closely resembles the Belgian clinical choice of treatment.
‡ In this column, the EUCAST breakpoint set for ‘pneumonia’ are used. EUCAST also has breakpoints for indications other than meningitis and pneumonia.
Creation and analysis of the database was done using Stata version 10 (StataCorp., USA). To determine significant trends over the period 2007–2010, the Cochrane–Armitage test was used. All tests were two-sided and P values ⩽0·05 were considered significant.
Overall, about 86% (n = 97) of all eligible Belgian laboratories participated, collecting a total of 12 635 invasive pneumococcal disease (IPD) isolates consisting of 555 (4·4%) isolates from meningitis patients and 12 080 (95·6%) isolates from bacteraemia cases. In the period 2007–2010 some hospitals merged which resulted in a lower number of reporting laboratories but similar national coverage was achieved.
Table 2 shows the resulting penicillin non-susceptibility proportion when different breakpoints were applied. The ‘BE EARS-Net’ breakpoint of a specific year represents the breakpoints in use in the NRL that year, and corresponds to pre-2008 CLSI breakpoints until 2009, and post-2008 CLSI breakpoints afterwards. By the old CLSI breakpoints, an average of 9·4% of isolates were classified as non-susceptible to penicillin, this fell by 94% to 0·6% when only the new CLSI breakpoints were applied. Using this ‘BE EARS-Net’ breakpoint set there was a strong and significant decreasing trend (P < 0·001) in the period 2007–2010 in the non-susceptibility proportions, as reflected in the EARS-Net reports. Closer examination of this trend showed that it was largely due to a sharp decrease, from 8·5% to 0·5%, between 2008 and 2009 which corresponds with the implementation of the new CLSI breakpoints. If only the old CLSI breakpoints are applied to this period, a much smaller decrease (8·5% to 6·5%) is seen. The 8% decrease reported by EARS-Net in 2008–2009 (8·5% to 0·5%) is in reality a 2% decrease as the remaining 6% are due to the change in breakpoints. There was no significant decreasing trend in the same period using the old CLSI breakpoints alone (P = 0·204), or the new CLSI breakpoints alone (P = 0·954). Applying the EUCAST breakpoints resulted in a penicillin non-susceptibility proportion fluctuating around 5%.
Table 2. Percentage trends of penicillin non-susceptibility of Belgian S. pneumoniae isolates using four different sets of clinical breakpoints

EARS-Net, European Antimicrobial Resistance Surveillance Network; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing.
* BE EARS-Net is not an actual breakpoint set: for a specific year it represents the breakpoints in use in the National Reference Laboratory that year, and corresponds to pre-2008 CLSI breakpoints until 2009, and post-2008 CLSI breakpoints afterwards, meaning the most up to date CLSI breakpoints were always used in Belgium for EARS-Net reporting.
† Statistically significant trend in the period 2007–2010 .
EARS-Net surveillance shows overall results which combine blood and CSF isolates. However, with new CLSI and EUCAST guidelines, these two types of isolates have very different breakpoints, therefore it makes sense to present them separately. In Table 2 both overall and separate results are shown. For CSF isolates there was no difference in non-susceptibility proportions between old CLSI, new CLSI or EUCAST breakpoints since the penicillin breakpoints are the same for meningitis (Table 1). On the other hand, for blood isolates there was a marked decrease of 99% in penicillin non-susceptibility proportions between old and new CLSI breakpoints (9·3% and 0·1%, respectively). The non-susceptibility proportions with EUCAST breakpoints fell in between these two values at 4·9%.
The difference in non-susceptibility proportions between blood and CSF isolates clearly depends on the breakpoints applied. When only the old CLSI breakpoints are used the difference between the two types of samples is not significant (1·2-fold, P = 0·099), but it is highly significant when the new CLSI breakpoints alone (76·2-fold, P < 0·001) or EUCAST breakpoints alone (2·3-fold, P < 0·001) are applied.
Surveillance of antimicrobial resistance is crucial for the control of resistance in pathogens. EARS-Net is one of the most useful of these surveillance systems due to its high degree of standardization, European coverage, high participation and long history. The 2010 annual EARS-Net report showed a significant decreasing trend for S. pneumoniae non-susceptible to penicillin in Belgium for the period 2007–2010 and this was due solely to the change in breakpoints in 2009. Without this change the proportion of these isolates would have decreased non-significantly from 9·4% to 8·7% (P = 0·204). Seventy-five percent of the sudden drop in non-susceptible isolates is explained only by the change in breakpoints, the remaining 25% representing a real change in susceptibility. The latter was no doubt influenced by other factors such as demographics of age or socio-economics [Reference Masiero5, Reference Karlsson6], antimicrobial consumption which is a major trigger for shifts in susceptibility patterns, and the prevalent serotypes of S. pneumoniae which may be highly influenced or ‘replaced’ by vaccination strategies [Reference Pletz7].
The new CLSI breakpoints pose a particular concern for the interpretation of EARS-Net data in its current form because they provide different breakpoints depending on the site of isolation (blood and CSF) as opposed to the combination of all invasive pneumococcal isolates favoured by this surveillance system. Based on the new CLSI breakpoints almost all blood isolates in this survey were susceptible to penicillin compared to 10–16% of non-susceptible CSF isolates, a difference of more than 70-fold.
Since the percentage of CSF isolates within the total group of isolates was very small, the overall proportion is more close to that of blood isolates. However, EARS-Net data suggest that the percentage of CSF isolates within the total group of isolates ranges from 0% to 43% between different countries [2]. This inconsistency in frequency and the very different non-susceptibility proportions between blood and CSF isolates with the new CLSI breakpoints results in a substantial limitation to the validity of the combined data for blood and CSF isolates as shown in EARS-Net reports. It should, therefore, not be used to compare non-susceptibility proportions between countries. With this in mind, we suggest that it would be useful to stratify the maps and tables of S. pneumoniae surveillance based on separation of CSF from blood isolates. It is noteworthy that the difference in non-susceptibility proportions between blood and CSF isolates is much smaller for EUCAST breakpoints (2·3-fold, P < 0·001) but would still justify separation of the source of isolates.
Previously, when the old CLSI breakpoints were used, blood and CSF isolates had non-susceptibility rates in the same range (9·3% vs. 11·4%, P = 0·099). Because many pneumococcal infections are treated on an empirical basis, this meant the EARS-Net data could be used for estimating the effect of empirical penicillin therapy for both meningitis and non-meningitis. However, the debate about the clinical sense of changing the breakpoints for S. pneumoniae has indicated that in the past many non-meningitis cases were unjustifiably classified as non-susceptible and therefore treated with broader spectrum antibiotics [8]. Thus the clinical utility of penicillin for bloodstream infections was underestimated when EARS-Net data were used to estimate the effect of empirical therapy.
It has also been suggested to continue to report data that apply the old breakpoint (0·06 μg/ml) in EARS-Net and other continuous epidemiological surveillance studies [Reference Weinstein, Klugman and Jones9]. An alternative would be to reanalyse all historical data with the new breakpoints. This would only be possible if MICs of all non-susceptible cases are known (as is the case for Belgium).
Different breakpoints between periods and countries are a reality of a surveillance system such as EARS-Net, and do not diminish their importance. However, results need to be interpreted in that context. The introduction of the same breakpoints in all European laboratories, one of the goals of the EUCAST project, could be a great step forward.
In this study we chose to apply EUCAST breakpoints for trend evolution limited to the 1·2 g × 4 subset, because this most closely resembles Belgian clinical practice (2–3 million international units four times a day); the new CLSI non-meningitis breakpoints are based on a higher daily dose regimen of 1·2 g × 6. This choice was made in order to predict the proportions of non-susceptibility in day to day practice, which are used in the Belgian EARS-Net data. European laboratories will be requested to switch to EUCAST breakpoints in the near future. EARS-Net will need to define which of the dosage regimens should be used in surveillance to avoid a situation where all laboratories use breakpoints from the same organization, but still do not use the same breakpoints. This work suggests that when the change occurs, a rise in non-susceptibility will be seen even if the microbiological situation remains the same. If the MIC distribution stays similar to the 2007–2010 data, non-susceptibility under the EUCAST breakpoints will be around 5% for Belgian EARS-Net reporting, while it would be <1% with the current (new CLSI) breakpoints. Such semantic discrepancy evokes confusion in a time-frame where appropriate choice of compound and dosing to minimize resistance development is encouraged and needed [Reference Drlica and Perlin10].
ACKNOWLEDGEMENTS
The authors thank all hospitals and clinical laboratories that participated in the related surveillance programmes.
DECLARATION OF INTEREST
None.




