Early in the morning of 16 December 2009, a villager at Nanshe village, Yangqu county in Shanxi province discovered his neighbour's dog frantically attacking and biting his flock of sheep (n=110) which were huddled in a sheepfold, a small cave of about 20 m2. The 3-year-old dog was immediately driven out. It died the next day at its home (about 100 m from the sheepfold) and its body was left frozen in the yard by its owner (temperature about −12°C). The dog attack had caused panic in the flock, resulting in crushing and trampling: 36 sheep, mostly baby lambs and young sheep, died over the next 2 days.
According to their owner, 2 weeks later some of the bitten sheep developed spasms, followed by paralysis and death within 2 days. The first three sheep were found dead on 1 January 2010, after which one or two deaths occurred almost every day following the appearance of rabies-like symptoms. After the initial outbreak the sheep owner immediately separated out more than 40 sheep with bite wounds (mostly on the face) and fenced them outside the sheepfold cave. A few dead sheep and several with severe disease symptoms were left in the cave. The remaining healthy sheep were transferred to another location and the owner reported the outbreak to the local veterinary administration.
On 3 January veterinarians from the Taiyuan Centre for Animal Diseases Control and Prevention visited the scene of the outbreak. Since the disease situation was worsening, with continuing deaths, and since rabies was suspected, the Diagnostic Laboratory for Rabies (DLR) at the Institute of Military Veterinary, Academy of Military Medical Sciences was contacted the next day. On 5 January, DLR experts arrived to find four paralysed adult sheep prostrate in the cave and four sheep carcasses frozen firmly to the ground. In the fenced-off area outside the cave, about a dozen of the 40 injured sheep were showing early symptoms of disease (cycling, moaning, chasing and mounting). Leashed to a tree trunk next to the fence, the sheep owner's 1-year-old dog was also showing early signs (malaise and anxiety with dull eyes, salivation, wariness and defensiveness). The owner claimed that his dog had also been bitten by the older dog. All three stages of rabies (prodromal, excitement, paralysis) were observed in the flock, including restlessness, moaning, hyperexcitation, sexual excitement, staggering or running with manic head butting, depression and lack of coordination. Once having fallen down, the sheep presented with arched back, tremors and a swimming movement of all four limbs. This was followed by paralysis and death. All infected sheep died within a few days of the disease developing, indicating that the fatality rate was 100%.
Saliva swabs were taken from the four paralysed sheep and the younger dog; three of the sheep and the dog were then destroyed and their brains removed. The brain of the first (elder) dog was also collected.
On 6 January the entire flock was humanely destroyed by the local veterinary administration as recommended by the National Directive for Control and Prevention of Rabies (amended, 2006) and the disease site fully disinfected to prevent possible disease transmission. The carcasses were buried deeply covered with a layer of lime. By the day of the slaughter, 2 weeks after the attack, 15 sheep (including the three paralysed ones mentioned above) had died of rabies.
Culling of infected sheep and dogs was in accordance with Ethical Guidelines for Animals in China and approved by the Ethical Panel of Shanxi Provincial Centre for Animal Disease Control and Prevention.
The investigation revealed that Nanshe is a village with 280 villagers in 90 families with 50 dogs allowed to roam free for the purpose of family protection. None of the dogs had ever been vaccinated against rabies. After the outbreak the residents of Nanshe and the nearby villages were ordered to have their dogs vaccinated.
In the laboratory, brain tissues were examined by fluorescent antibody test (FAT) and RT–PCR followed by sequence analysis. Saliva specimens were tested only by RT–PCR. Rabies virus from the brain of the elder dog was isolated by mouse intracerebral inoculation and designated SXTYD01. RT–PCR was performed using a previously described protocol [Reference Jiang1]. The rabies FAT and mouse inoculation test procedures were based on WHO protocols [Reference Meslin, Kaplan and Koprowski2] using brain tissue and FITC-conjugated anti-rabies monoclonal antibody (Fujirebio Diagnostics Inc., USA). The use of mice for inoculation procedures was in accordance with Ethical Guidelines in China and assessed by the Ethical Assessment Panel of the Institute of Military Veterinary. Results showed that all five brain tissues tested (two dogs, three sheep) were positive by FAT and RT–PCR. Of five saliva specimens, only one showed specific N gene amplification of rabies virus by RT–PCR. For virus isolation a litter of suckling mice was inoculated intracerebrally with a suspension of brain tissue from the first dog, and all died of rabies on days 13–14 post-inoculation. Brain smears of each dead mouse were also confirmed FAT positive. All animal brain samples gave clear amplification of an 845-bp N gene fragment (55-899 nt) of rabies virus. The PCR products of the first dog and a sheep were sequenced by a commercial sequencing service (Takara Ltd, China), and both sequences were 100% identical. The sequence from the dog isolate SXTYD01 has been submitted to Genbank (accession no. JF441064). The above results confirm that the outbreak was caused by rabies virus. Temporal considerations indicate that the first dog to die was the index animal and had transmitted the infection to the sheep and to the second dog.
Canine rabies is common in China, and spillover of rabies from dogs to other domestic animals, such as pigs, has been reported sporadically [Reference Jiang1]. Sheep rabies has been reported in other countries, such as Mali [Reference Dao3], Brazil [Reference Sato4] and Denmark [Reference Tjornehoj5], caused not only by rabies virus [Reference Dao3, Reference Sato4] but also by European bat lyssavirus type 1 (EBLV-1) [Reference Tjornehoj5]. Unlike the large outbreak reported here, the sporadic outbreaks of sheep rabies in these countries have involved only a few animals.
The clinical symptoms of rabid sheep infected by rabies virus have not been adequately described. The existing information comes from natural and experimental infection with EBLV-1, which includes aggression, wool chewing, head butting, drooling, restlessness, depression, muzzle and head tremors, sexual excitement and mounting, lack of coordination and paralysis [Reference Tjornehoj5, Reference Brookes6]. Most of these symptoms were also observed in the present outbreak except that increased salivary secretion was not apparent. In addition, moaning, arching of the head and swimming motions of the limbs were commonly seen in the later stages. Biting is not only a common sign of rabies but also the most important means for rabies virus to maintain its natural circulation and transmission in animals. In the outbreak described here, aggressive sheep–sheep biting behaviour was not observed, indicating that sheep-to-sheep transmission was unlikely. This differs from pig rabies, since transmission of infection through pig–pig biting has been reported to play an important role in exacerbation of rabies outbreak in a pig population [Reference Jiang1]. The current outbreak also showed that the incubation period of infection was about 2 weeks for sheep with bites on their faces, after which the disease developed rapidly. Following onset of symptoms, infected sheep usually died within 2–5 days and most of the face-injured sheep developed the disease, indicating that sheep are highly susceptible to rabies infection and that a dog bite on the face is a very efficient transmission route. The short and uniform incubation was also observed in a clinical investigation which showed that human rabies cases bitten by rabid wolves on the face and neck had shorter incubation (3–4 weeks) and higher mortality (60%) than those with bites on limbs or trunk [Reference Baltazard7].
To examine the question of viral shedding from the mouth, the saliva samples of the secondary-infected dog and the four moribund sheep were each tested twice by RT–PCR. Only one sheep saliva sample was positive, indicating only limited oral viral shedding, which is consistent with the observation that intermittent shedding of rabies virus in the saliva of animals clinically manifesting disease symptoms is a common phenomenon [Reference Delpietro8–Reference Fekadu10].
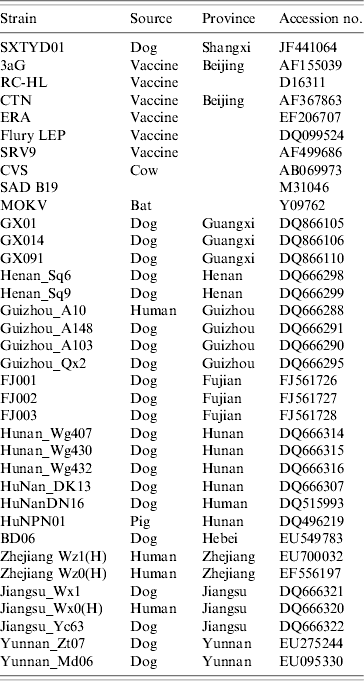
China has the second highest incidence of human rabies worldwide due to dog bites [Reference Tang11]. Shanxi province is not a region of high rabies prevalence in China, however, and no human or animal rabies had been reported in Yangqu county in recent years. The source of transmission is unclear. Nevertheless, it is a fact that in this region most families have at least one dog and that these animals are unvaccinated and allowed to roam freely. Since there was 100% identity between the partial N gene sequence of virus from the index dog and one of the affected sheep, it seems clear that rabies transmission was from this dog to the sheep. Comparison with N gene sequences of other Chinese isolates (GenBank; Table 1) revealed that isolate SXTYD01 segregated within subgroup A (Fig. 1). It showed the highest nucleotide identity (99·9%) with BD06, a street virus isolated in 2006 from a rabid dog in Baoding city, Hebei province, an eastern neighbour of Shanxi and about 300 km from Yangqu. This provides a possible association of this sheep rabies outbreak with a spread of BD06, although this transmission route has not been established.
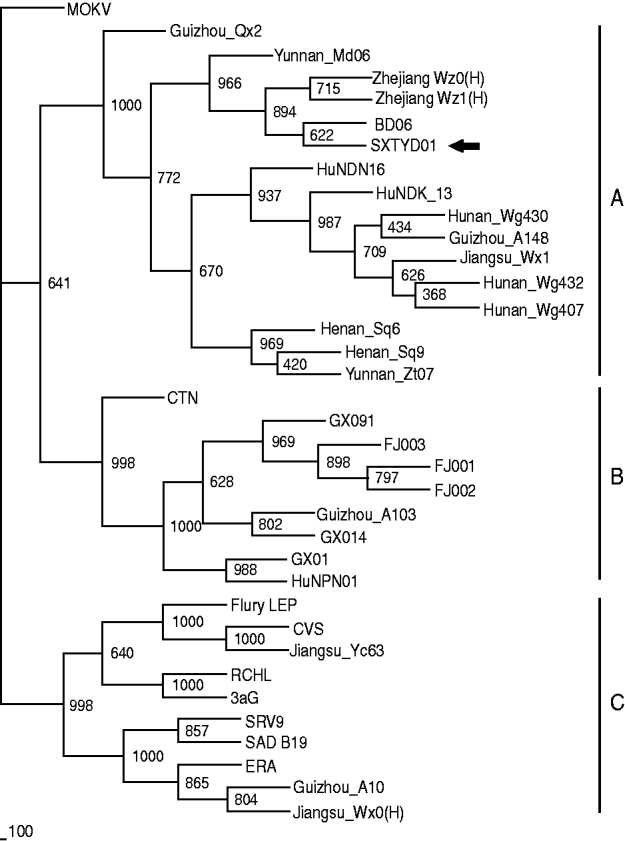
Fig. 1. The 804 bp sequence of the N gene (77-880 nt) was used for phylogenetic analysis within rabies virus, which was conducted using PHYLIP version 3.63 (USA) by the maximum parsimony method. The tree was estimated statistically by 1000 replicates of the bootstrap value and visualized by the Treeview program (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). MOKV was used as the outgroup. The nomenclature of subgroups A, B and C is that referred to in a previous publication [Reference Jiang1].
Table 1. The 36 strains or isolates of rabies viruses used in the study
Our observations strongly support the conclusion that the Yangqu sheep rabies outbreak was caused by a rabid dog. Rabies is a zoonotic disease with an important impact on public health. Spillover of rabies virus from dogs and wild reservoir animals leads to more than 55 000 human deaths every year [12] and also imposes severe economic losses on the livestock industry [Reference Gomes13]. The destruction of the entire sheep flock due to the rabies outbreak lead to a significant economic loss, emphasizing the importance of dogs in transmission of rabies in China; dog control, as well as vaccination, particularly in rural areas, needs to be better implemented to prevent rabies epidemics in dogs, domestic animals and human populations.
ACKNOWLEDGEMENTS
This work was supported by the following grants to C.T.: Special Fund for Agro-scientific Research in the Public Interest (No. 201103032), National Key Technology R&D Programme (Grant No. 2010BAD04B01) and Joint Project of NSFC/Yunnan Province (U1036601).
DECLARATION OF INTEREST
None.




