INTRODUCTION
Rubella is an infectious viral illness of childhood that can be so mild (or even asymptomatic) that most adults will be uncertain of their infection history. It can also be confused with other infections such as parvovirus B19. Prior to immunization programmes, rubella had a worldwide distribution occurring sporadically and epidemically in temperate climates, mostly in spring. Infection occurs most commonly in children and the resulting natural immunity is probably lifelong [Reference Lee and Bowden1]. In pregnant women, the risk of intrauterine transmission is up to 90% if infection occurs in early pregnancy (8–10 weeks' gestation), when the viraemia leads to placental infection and spread of the virus, which causes a chronic infection of the foetus leading to the development of congenital rubella syndrome (CRS) [2–Reference Banatvala and Brown5].
Early in 1960, three rubella vaccines were licensed, with a live attenuated vaccine based on the RA27/3 strain dominating. This strain is now included in the measles, mumps and rubella combined vaccine (MMR). In 1970, the USA adopted a policy of universal rubella vaccination for pre-school children [Reference Preblud6]. In contrast, at the same time, Australia, the UK and parts of Europe initially adopted a selective programme aimed at pre-pubertal girls, female nurses and teachers who were deemed to be at risk of acquiring rubella, and may not have acquired it naturally before puberty. Later recommendations advised the screening of pregnant women for rubella immunity and the offer of immunization postpartum if considered susceptible [Reference Tookey and Peckham7, Reference Tookey8]. In 1988, the UK introduced the MMR vaccination for all children aged 12–15 months, resulting in a fall in the number of cases of rubella. The incidence of CRS fell from an average of 48 births and 742 terminations between 1971 and 1975 to an average of four births and nine terminations between 1991 and 1995 [Reference Tookey8]. In 1994, to combat a predicted measles outbreak, all school children (aged 5–16 years) were offered a single dose of measles and rubella combined vaccine (MR) [Reference Tookey8], which caused the rubella susceptibility rate in children aged 5–16 years to fall to 3·7% [Reference Miller9]. Despite this, the incidence of rubella infection, confirmed by laboratory testing, rose in 1996. However, these cases were mainly males in the 17–24 years age group that had not been included in the 1994 MR campaign. There was very little impact on the antenatal population, who in 1996 had a rubella susceptibility rate of 1·2% (parous) and 2% (nulliparous) [Reference Miller9]. Rubella vaccination of teenage girls was discontinued in 1996 and replaced by a second dose of MMR for pre-school children aged 4–5 years [Reference Tookey and Peckham7].
Generally, the levels of reported rubella in the UK are low, with no confirmed cases of rubella in Wales since 2005 (see HPA database [10]), and fewer than ten cases in 2009 in the UK (see HPA database [11]). Similarly, incidence rates of CRS in the UK are low. Three cases of CRS were reported in the UK between 2005 and 2007 and none in 2008 [12]. Despite this, changes to vaccination strategies, and the variability of MMR uptake rates over the last 10 years, make it important to get a picture of the rubella status of pregnant women in the UK. In this study, we provide data on rubella antibody levels for 11 987 women in one NHS Health Board area in South Wales between the years 2005 and 2009.
METHOD
Routine blood tests are undertaken for all pregnant women receiving antenatal care; normally between 11 and 13 weeks of pregnancy. All blood screening request forms from pregnant women attending the Royal Glamorgan and Llwynypia hospitals were examined for the period 2005–2009 (3 years retrospective and 2 years prospective collection) and age, gravida and stated immunization history was recorded. The immunization history was from personal or maternal recall and was not used in analysis as studies have shown that personal/maternal recall of immunization history is unreliable [13, Reference Khare14]. Rubella testing was performed using the Diasorin ETI-Rubek ELISA kit (Diasorin, Italy) on an automated ELISA system, according to manufacturer's instructions. The calibrators for the assay are 0·0, 10, 25, 50 and 200 IU/ml, and a graph is generated which gives accurate readings at the lower end down to 0 IU/ml. The WHO now recommends a level of >10 IU/ml as a definition of immunity [Reference Cutts15]. The cut-off used in this laboratory, in line with international guidelines, is 10 IU/ml, i.e. <10 IU/ml is classed as susceptible, and ⩾10 IU/ml is classed as immune [Reference Cutts15, Reference Dimech16].
All tests with results <12 IU/ml were repeated using the Abbott AxSym automated analyser (Abbott Diagnostics, USA) according to the manufacturer's instructions (2005–March 2009) and bioMérieux Vidas (France) (April–December 2009). The laboratory takes part in the NEQAS quality assurance scheme and consistently has results above the mean for UK laboratories. All kits were CЄ (Conformité Européene) marked, having been standardized to the WHO international rubella virus serum [Reference Dimech16]. In cases where one result was ⩾10 IU/ml and one <10 IU/ml, the lowest result was reported in clinical practice. Attempts to standardize rubella status reporting have recently been made, one 2007 study examined results of antibody testing performed by reference laboratories in 21 countries [Reference Tischer17]. Researchers defined a cut-off of <4 IU/ml as seronegative, 4–7 IU/ml as equivocal and >7 IU/ml as seropositive. It was stated, however, that for women of childbearing age the defined cut-off for immunity should be an antibody titre >10 IU/ml [Reference Tischer17, Reference Skendzel18]. The Diasorin assay used in this laboratory was used by four of the 21 reference laboratories and gave 100% agreement on testing the negative samples but an underestimation of antibody in the positive samples [Reference Tischer17]. A 2008 study of eight EIA kits including the Diasorin, Abbott and bioMérieux kits demonstrated that these kits had comparable sensitivity and specificity with no false-positive results [Reference Dimech16].
For the purposes of this study, therefore, we elected to use a cut-off of 10 IU/ml to define immunity, and to avoid possible exaggeration of the results only those sample where both tests were <10 IU/ml were recorded. Where both test results were <4·0 IU/ml individuals were recorded as seronegative. Data was analysed by calculating the percentage of pregnant women screened in this area who presented with rubella IgG antibody level of <10 IU/ml and <4·0 IU/ml for each year of the study (2005–2009), as well as for first and second (or subsequent) pregnancies. Trend analysis was conducted using the Statcalc component of Epi Info v. 6.0 (CDC, USA), and involved χ2 calculations for linear trend to see if a significant trend existed for increasing rubella antibodies across the five study years [Reference Bland19, Reference Campbell20] for all, first and second (or subsequent) pregnancies. In cases where no gravida was stated, data were included in the overall susceptibility calculations, but were omitted from first or subsequent pregnancy data.
A separate analysis was undertaken for those born before 1983, and those born between 1987 and 1992. Figures were also extracted for those receiving the offer of differing immunization programmes; those born before 1983 were offered rubella vaccine pre-puberty, those born between 1987 and 1992 were offered one-dose MMR vaccine, an MR vaccine in 1994 and a second MMR in the catch-up programme of 2005, and those born after 1992 had the standard MMR programme [Reference Tookey and Peckham7, Reference Tookey8].
RESULTS
Of 11 987 pregnancies screened, 4026 (33·6%) were first pregnancy and 7254 (60·5%) second or subsequent pregnancy. In 706 (5·9%) cases no gravida was stated. The results are shown in Table 1. The results in Table 1 show a non-statistically significant trend for a decrease in those with those with rubella antibody levels of <4·0 IU/ml (defined as seronegative) for this period from 29/2312 (1·3%) in 2005 to 21/2447 (0·9%) in 2009 (χ2 for linear trend=0·279, P=0·56). In contrast there is a significant increase in those with rubella antibody levels <10 IU/ml for this period from 88/2312 (3·8%) in 2005 to 124/2447 (5·1%) in 2009 (χ2 for linear trend=10·27, P=0·001).
Table 1. Antenatal blood screening results for rubella IgG at the Royal Glamorgan and Llwynypia hospitals: 2005–2009
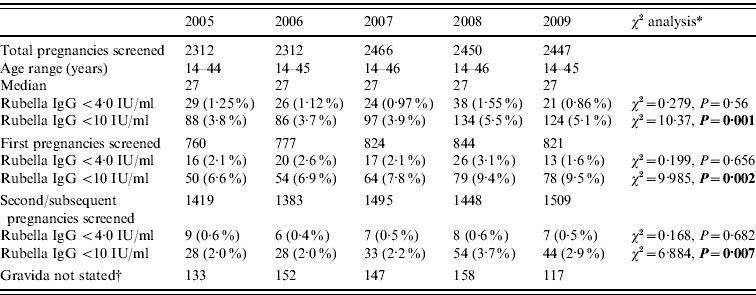
* Statistically significant results reported in bold.
† Figures not entered into the statistical trend analysis of the first and second pregnancy data.
Analysis by cohort of women depending on their vaccination experience, looking at those born prior to 1983 who were offered rubella vaccine pre-puberty at a time when wild rubella virus was still in common circulation, and those born after 1983, who were offered the childhood vaccination programme or a catch-up childhood immunization shows that the proportion of first pregnancies with titres <4 IU was 1·1% (21/2002) compared to 3·4% (69/2022) in those born after 1983 (χ2=25·176, P<0·0001) and 2·2% (44/2002) for titres <10 IU compared to 14·0% (282/2022) for those born after 1983 (χ2=171·43, P<0·0001).
The cohort of mothers born between 1987 and 1992 were offered a range of immunization opportunities from a single MMR, to a school catch-up and then the local catch-up campaign for school children and young adults. The results of this cohort are shown in Table 2, and show a decline in the proportion of this group with rubella levels <4 IU/ml and <10 IU/ml.
Table 2. Antenatal blood screening results for rubella IgG in those born 1987–1992 offered a single MMR plus catch-up immunization the Royal Glamorgan and Llwynypia hospitals: 2005–2009
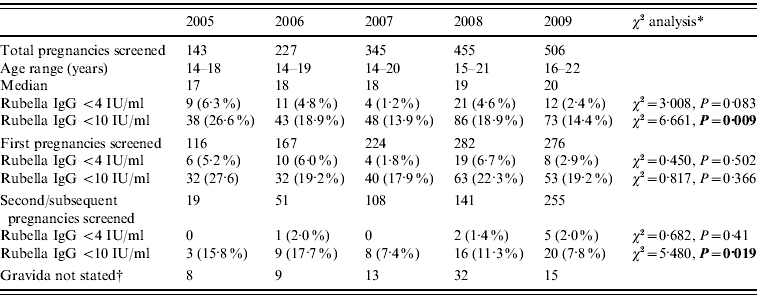
* Statistically significant results reported in bold.
† Figures not entered into the statistical trend analysis of the first and second pregnancy data.
No women born 1992 onwards were included in this study in 2005 or 2006; six, 30 and 60 were identified in 2007, 2008 and 2009, respectively. Of these 96, almost a third (29/96) had rubella IgG antibody levels of <10 IU/ml and >10% (10/96) had rubella IgG antibody levels of <4 IU/ml.
DISCUSSION
Overall, the results of this study show that there has been a significant increase in those with rubella antibody levels <10 IU/ml from 88/2312 (3·8%) in 2005 to 124/2447 (5·1%) in 2009 (χ2 for linear trend=10·27, P=0·001). The proportion with a level <4 IU/ml has not changed significantly over this time. However, this cohort includes both women who were born prior to 1983 and who would have been offered pre-pubertal immunization with rubella, and those offered childhood immunization. The results in these two cohorts are very different; those born before 1983 have very low levels of seronegativity (<4 IU/ml) and high levels of immunity (>10 IU/ml). This may reflect that fact that a high proportion of this group were probably infected with rubella in childhood, as the immunization programme at that time did not prevent the circulation of rubella in the community, and it is well recognized that natural immunity to rubella gives higher titres than vaccine-induced immunity.
In those born after 1983, the prevalence of those with rubella antibody levels <4·0 IU/ml did not change significantly over time (detailed data not shown), from 2·2% in 2005 to 1·8% in 2009 (χ2 for linear trend=0·001, P=0·982) and the rubella levels <10 IU/ml remained steady at 11% in 2005 and 9% in 2009 (χ2 for linear trend=1·43, P=0·23). The levels of seronegativity, which reflect non-vaccination or low titres following immunization, and of titres <10 IU/ml is higher than the pre-1983 cohort. There seem to be changing patterns within these cohorts, with the 1987–1992 cohort showing a decline in levels of antibody <4 IU/ml and <10 IU/ml over the time period, but with very high levels of titres <10 IU/ml in the (albeit small) post-1992 cohort. This may reflect the success of the 2005/2006 catch-up MMR campaign in Wales (see below).
Rates of titres of <4 IU/ml and <10 IU/ml for the second or subsequent pregnancies are lower than those for first pregnancy as would be expected with postpartum immunization programmes. However, despite a policy of routine antenatal testing and postpartum MMR vaccination, seven (0·5%) of the women attending for their second pregnancy in 2009 had rubella IgG antibody levels of <4 IU/ml and 44 (2·9%) had rubella IgG antibody levels of <10 IU/ml. Further work is underway to determine if this is due to system failure in vaccine delivery, or lack of response. However, in the last two quarters the antenatal services in this area failed to meet the 95% target for postpartum immunization of women identified as having rubella IgG antibody level <10 IU/ml [21]. It is generally difficult to obtain accurate figures for uptake of postpartum immunization rates as there is no requirement to notify adult MMR immunizations to a recording authority. One study showed that the uptake can vary between 5% and 80% [Reference Vyse22] and in an English study conducted in the East of England and the West Midlands the uptake was 29% and 60%, respectively [Reference Yung, Skidmore and Lord23].
The high number of women born after 1983 with rubella IgG antibody level <10 IU/ml is noteworthy. This level of response may reflect low levels of immunization or lack of response to vaccination. In 1998, uptake of the MMR vaccine fell due to adverse publicity about its safety but is slowly increasing [24]. Uptake rates for MMR in Rhondda Cynon Taff, (of which the Health Board is the larger part), have increased from about 83% in 2003/2004 to 90% in 2008/2009 for first vaccination and 80% for second vaccination uptake, still well below the level required for herd immunity [24]. A MMR catch-up programme was undertaken in Wales in 2005/2006, but although immunization was received by 53 798 secondary school students and 7112 college or university students, over 73 000 identified as in need of at least one dose of MMR did not accept immunization [Reference Gibbons25]. This cohort will shortly be reaching childbearing age.
The overall prevalence of antibody levels of <10 IU/ml is comparable to that recently reported from Sweden, where 95·5% of a cohort of 39 890 antenatal sera screened between 2004 and 2006 had levels >10 IU/ml. The Swedish immunization programme differed from that in the UK in that the pre-pubertal rubella vaccine was replaced in 1982 by two doses of MMR at 18 months and 12 years [Reference Kakoulidou26]. Examination of primiparous women in this cohort demonstrated that of those born prior to 1980 who were routinely offered the monovalent rubella vaccine at age 12 years and were exposed to the wild virus still circulating in the community, 2·7% demonstrated levels of <10 IU/ml, compared to 8·7% of those offered two doses of MMR vaccine after 1982. This study also found higher levels of titres of <10 IU/ml in those offered the childhood vaccination programmes. We are currently studying in greater detail the immunizations received by those individuals with low titres of rubella.
A key concern is whether an antibody titre of <10 IU/ml represents a risk of rubella acquisition should exposure occur. In their experimental study, O'Shea et al. demonstrated that 1/19 (5%) of those with a level <15 IU/ml (vaccine induced) and 8/10 (80%) of seronegative women (<1 IU/ml) could acquire infection if exposed [Reference O'Shea, Best and Banatvala27]. They also found that if low level immunity was due to past infection it was unlikely that infection would occur. It must be stressed that this study was performed using nasal challenge with the vaccine and may be of limited application in practice. As there has been no evidence of circulating rubella in the study area for many years it is unlikely that low-level immunity (1–10 IU/ml) in those born after 1992 is due to past infection. Applying O'Shea's figures to data from our study would suggest that that in 2009, 13 women had levels of <4 IU/ml, and if considered seronegative, could potentially be infected during pregnancy in one year in the Trust area if the rubella virus was circulating. However, the low prevalence of titres <4 IU/ml make it unlikely a large outbreak would occur in South Wales.
While current figures show very low levels of circulating rubella in the UK, it is not only the risk of exposure within the UK that should be considered. Many European countries have varied immunization programmes and coverage. For example, parts of Italy only have around 26% vaccine coverage and an elimination plan for rubella was only implemented in 2003 [Reference Bonanni28]. A 2004 Greek study found that 23·2% of women of childbearing age were susceptible to rubella [Reference Gioula29]. Most Eastern European countries have introduced rubella/MMR vaccine in the last 5–10 years, but despite this Poland reported 20 000 cases of rubella in 2007 (see the Euvac database [30]). Finally, in South Africa, a country which has hosted several international sporting events in 2010, the measles vaccine is given as routine, but mumps and rubella are not part of the routine immunization schedule and rubella is not a legally notifiable disease, although 1072 cases were reported in 2007 (see WHO database [31]). Low immunization rates and circulating rubella virus in countries such as Greece, Italy and South Africa pose a potential risk to pregnant holidaymakers, particularly given that investigations of cases of CRS showed that a high percentage of mothers had acquired the infection abroad [Reference Tookey and Peckham7, Reference Mehta and Thomas32]. For this reason, MMR vaccine is recommended by the HPA for travel to areas where prevalence is high and/or vaccine coverage is low (see HPA database [33]). In addition, there is a risk of importing rubella from these countries.
Although undertaken in one Trust area in South Wales, it is likely that these results can be generalized to other areas, as immunization schedules and the decline in uptake of MMR are UK issues [34]. If anything, the data presented for this area may under-represent rubella susceptibility in other populations in the UK, as it is known this is higher in immigrant communities [Reference Tookey8]. For example, Sri Lankan women living in Britain have rubella susceptibility rates between 15% and 23% [Reference Tookey, Cortina-Borja and Peckham35, Reference Thomas and Mehta36]. It was not possible to determine ethnicity for this study from the antenatal booking request forms but the 2001 census figures for the Rhondda Cynon Taff County Council area, of which the study area is a part, showed that 97·5% of the population were white British and 0·5% were white Irish, only 2% being in the ‘other’ category (see ONS database [37]). Given this, it seems quite possible that levels of seronegativity and low antibody levels (i.e. <4 IU/ml and <10 IU/ml) may be higher in areas where there is a higher percentage of mothers born overseas.
This study has demonstrated that there are changing patterns of rubella seronegativity and susceptibility in pregnant women born before and after 1983. The increase in the number of young women with levels of <10 IU/ml requires further monitoring, particularly as the cohort experiencing low levels of MMR uptake in the late 1990s reaches childbearing age. Although to date the number of cases of CRS reported in the UK remain low and outbreaks of rubella are unlikely, these levels suggest there is potential for infection to be acquired or imported from abroad.
ACKNOWLEDGEMENTS
We thank the staff at the Virology Laboratory, Royal Glamorgan Hospital for performing almost 12 000 rubella IgG antibody tests, and are grateful for the input of anonymous referees whose helpful comments significantly improved the manuscript.
DECLARATION OF INTEREST
None.




