INTRODUCTION
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are a class of drugs designed to lower lipid levels [Reference Jain and Ridker1]. The benefits of these drugs for persons with cardiovascular disease are well established. A large meta-analysis of 90 056 individuals enrolled in 14 randomized controlled trials showed that, over a 5-year period, statins were associated with a 21% reduction in major cardiovascular events, including a 19% reduction in mortality associated with coronary events and a 17% reduction in fatal or non-fatal stroke [Reference Baigent2]. The potential of immune modulating drugs, in particular statins, in the treatment and prophylaxis of influenza has been succinctly summarized by Fedson who has also raised the question of their use in a pandemic especially in countries where antiviral drugs and vaccines might not be available [Reference Fedson3]. As well as reducing levels of low density lipoprotein cholesterol, statins have anti-inflammatory, antioxidant, immunomodulatory, anti-apoptotic, anti-proliferative, anti-thrombotic, and endothelium protecting features (collectively referred to as pleiotropic effects) [Reference Jain and Ridker1–Reference Schonbeck and Libby7]. The use of statins as supplements to appropriate disease specific therapy has become almost mandatory in a range of cardiovascular and related diseases [Reference Baigent2, Reference Colhoun8, Reference Collins9]. Statins may also be beneficial in preventing and treating patients with life-threatening infections associated with cytokine dysregulation including bacterial sepsis [Reference Almog10–Reference Liappis13]. The ‘cytokine storm’ phenomenon associated with severe influenza is a particular reason why statins have been considered potentially protective against severe influenza [Reference Cheung14].
There have been several recent studies describing the effect of statin therapy on pneumonia and pneumonia-related mortality [Reference Frost15–Reference van de Garde19]. Two of these studies support the use of statins to reduce the risk of pneumonia [Reference Schlienger18, Reference van de Garde19]; two provide evidence of reduced pneumonia-related mortality [Reference Majumdar16, Reference Schlienger18] and one, based on persons admitted to hospital with community-acquired pneumonia was inconclusive [Reference Majumdar16]. Smeeth and colleagues studied a statin-user population in relation to a range of health outcomes in 129 000 subjects included in a UK general practice database between 1995 and 2006 [Reference Smeeth20]. They observed a small reduced risk of pneumonia but no effect on other respiratory or urinary tract infections. All these studies recognized the importance of confounding variables particularly the ‘healthy user effect’ [Reference Fedson3]. Differences in study design (prospective and retrospective cohorts, case-control), definitions of statin use and confounding variables limit the ability to combine the results, but in general they point to favourable effects in persons with serious respiratory infections possibly because of the primary cardiovascular benefits. We aimed to examine the effect of statin use on the incidence of acute respiratory infections in persons aged ⩾45 years presenting to general practitioners (GPs). There have been no randomized control studies in which this problem has been investigated.
METHODS
A population-based retrospective cohort study design was employed which included eight winter influenza seasons (1 July 1998 to 30 June 2006). Information on all variables including outcomes was derived for each patient for each study year on persons aged ⩾45 years registered in the practice at the onset of the year. Study years ran from 1 July to 30 June the following calendar year; thus each study year included a whole UK influenza season. The study protocol was approved by the West Midlands Research Ethics Committee.
We calculated a sample requirement of 22 000 person-years to detect a relative risk of 0·8 of developing an acute respiratory infection for statin users compared with non-users, with 80% power at the 5% significance level. It was assumed that over the study period the average annual prevalence of statin use was 9%, whilst the annual incidence of acute respiratory infection amongst non-statin users in the study group was assumed to be 10%. This was a minimum estimate and took no account of the non-independence of observations, of analysis in individual years or of analysis for more specific respiratory diagnoses.
We used data collected in selected practices participating in the Weekly Returns Service (WRS) of the Royal College of General Practitioners (RCGP) [Reference Fleming21]. The selection (25/102 practices) was based on the availability of a comprehensive electronic morbidity and prescribing dataset available over the entire study period, computer software amenable to remote interrogation of anonymized data, and willingness of the practice to collaborate. The practices concerned, in common with the majority of practices in the UK, use electronic records exclusively to record all aspects of medical consultation and interventions. Relevant morbidity and prescribing data are stored as Read codes which facilitate automated analysis [Reference Chisholm22]. Recognizing that statins were rarely prescribed to young persons, we restricted data extraction to persons aged ⩾45 years for whom there was a continuous period of registration within the same practice for a minimum of 4 years at the endpoint of each winter season analysis. The records examined were those of persons who had received a prescription for a statin, or likely to have been considered for long-term statin therapy. Persons (aged ⩾45 years) with cardiovascular diseases (including stroke and hypertension), diabetes, or hypothyroidism were included. There were no exclusions from data extraction routines of persons aged ⩾45 years although persons with a diagnosis of cancer, HIV/AIDS, organ transplantation or receiving prescriptions for immunoglobulin or immunosuppressant drugs (including oral prednisolone) were excluded from the main analyses in each year after diagnosis. Data extraction included Read code entries and their linked date describing all morbidity diagnoses with their episode type (distinguishing new from ongoing consultations) [Reference Fleming and Elliot23]; prescriptions issued for selected drugs [statins, glucocorticoids, corticoinhalers, non-steroidal anti-inflammatory drugs (NSAIDS), aspirin, gastric acid suppressants and immunosuppressants]; vaccination with pneumococcal and influenza vaccines. Smoking status and body mass index data were also extracted, but the latter was not used in the analysis because of incomplete recording. Persons first diagnosed with chronic conditions during the study period [mainly the recruitment conditions and chronic obstructive pulmonary disease (COPD)] and persons receiving a pneumococcal vaccination were analysed as such for the remainder of the study period: influenza vaccination was considered in a season-specific manner.
Individual outcomes of interest were influenza-like illness (ILI), acute bronchitis, pneumonia, upper respiratory tract infections (URTI), acute respiratory infections combined (ARI which includes ILI, acute bronchitis, pneumonia and URTI). Urinary tract infections (UTI) and herpes zoster were included as potential ‘comparator’ illnesses where we expected the potential effect of statins might be less because the host cytokine response is likely to be less than that associated with influenza.
Patients were assigned to a statin-user status for each study year (influenza season):
Regular user: those who had at least two prescriptions of any statins prior to 31 December in the study year and at least one prescription after 31 December;
Possible user: those who had at least one prescription of any statin prior to 31 December in the study season;
Non-user: those with no recorded statin prescription prior to 31 December. The issue of other drugs used in the analysis was also considered specifically in relation to the year studied.
Age (45–54, 55–64, 65–74, 75–84, ⩾85 years) was determined at 31 December in each study year. The number of new episodes of illness for diagnoses in each study year, other than the recruitment diagnoses, was included in analyses as a measure of the propensity of patients to consult.
Analysis
Statistical analyses were performed with Stata version 10 [24]. We examined the effects of statin use in relation to outcome, separately for each outcome, and stratifying by influenza vaccination status (vaccinated at least 14 days prior to outcome event thus allowing time for the development of immunity; non-vaccinated). The explanatory variables used in the logistic regression models are shown in Table 1.
Table 1. Explanatory variables used in logistic regression models with given outcome when stratified by influenza vaccination
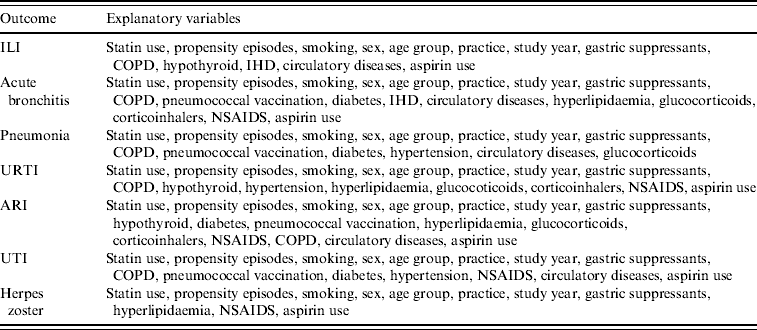
ILI, Influenza-like illness; URTI, upper respiratory tract infection; ARI, acute respiratory infections combined; UTI, urinary tract infection; COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; NSAIDS, non-steroidal anti-inflammatory drugs.
Hierarchical (nested) logistic regression models were used to account for non-independence of observations due to having repeated measurements over time from individual patients, and clustering of patients in GP practices. Random effects at patient level were incorporated into models; models were also adjusted for fixed effects of GP practice. Adjusted risk estimates were expressed as odds ratios (ORs) and 95% confidence intervals (CIs) with Wald test P values quoted for the ORs. Differences in the effect of statin use on each of the outcomes in the 8 years studied were investigated by having a single interaction term in non-hierarchical logistic regression models between statin use and year, without any stratification but allowing for clustering on patient. Wald test P values were used to evaluate statistical significance of the interaction.
Finally, we were interested in the effects observed in outcomes during a critical influenza active period in the millennium winter (week 48/1999 to week 5/2000) in which there were particularly high numbers of persons in England seeking hospital admission because of respiratory infections. A benefit in such a situation, regardless of what happened in other years would bear disproportionately on any subsequent prescribing recommendations. The period was determined from virological reports submitted to the HPA and included 70% of the influenza-positive swabs reported in the entire winter [Reference Fleming25].
RESULTS
The study is based on 329 881 person-year observations (patients aged ⩾45 years; 40 000–50 000 persons in each year) in which about 18% were classified regular statin users. Table 2 shows that use is greatest in the 65–74 years age group; the proportions receiving influenza and pneumococcal vaccination were greater in the regular user than non-user group. There are higher statin-user than non-user proportions of persons with ischaemic heart disease (IHD) and with diabetes, but lower with COPD. The proportions of users and non-users reporting each of the outcome diseases are broadly similar (results relating to possible statin users are available on request but are not presented here nor considered further because they do not contribute to the interpretation of the findings).
Table 2. Number of person-years (%) for statin-use status
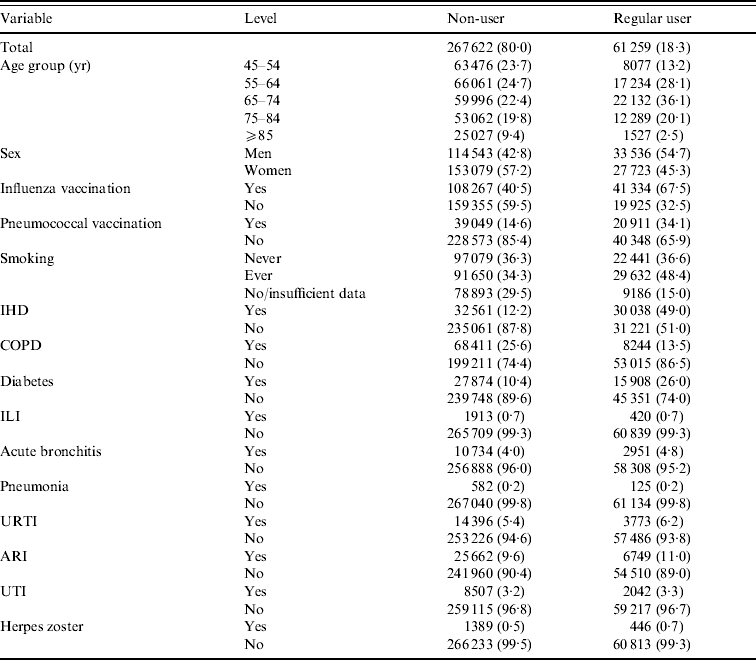
IHD, Ischaemic heart disease; COPD, chronic obstructive pulmonary disease; ILI, influenza-like illness; URTI, upper respiratory tract infection; ARI, acute respiratory infections combined; UTI, urinary tract infection.
Forty-six percent of the study population (person-years) had received influenza vaccine at least 14 days previously and 18% received pneumococcal vaccine in the previous 5 years (data not shown). Both these proportions were highest in the 65–74 years age group. Regular statin use in the total population was higher in both the influenza and pneumococcal vaccinated than non-vaccinated groups and also in the subgroups with IHD, COPD and diabetes.
Table 3 shows the adjusted ORs for statin users vs. non-users according to influenza vaccination status and demonstrates that non-vaccinated statin users have higher risks than vaccinated statin users for all outcomes except UTI.
Table 3. Adjusted odds ratios for statin users vs. non-users according to influenza vaccination status
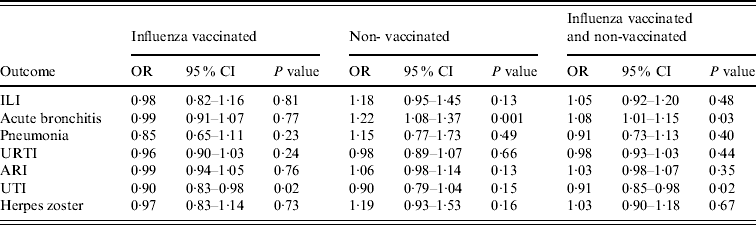
OR, Odds ratio; CI, confidence interval; ILI, influenza-like illness; URTI, upper respiratory tract infection; ARI, acute respiratory infections combined; UTI, urinary tract infection.
We repeated the primary analysis using the same outcome endpoints during a critical influenza active period in the millennium winter (week 48/1999 to week 5/2000). However, the results of this analysis were little different from those reported above (and thus not presented); in particular they did not disclose any evidence of protective effects from statin use during this especially severe influenza season.
The interaction between year of study and statin use is illustrated (Table 4) showing data for the largest and smallest adjusted ORs in those cases where the corresponding interaction was significant (here P values <0·01 are deemed significant). For all outcomes, there were significant differences in the effect of statins between the years. As an example: for ILI, users in 2005–2006 had an OR of 0·54 (relative to non-users in 1998–1999) and non-users in 2004–2005 had an OR of 1·58 (same baseline). Differences in one or more of the ORs (extreme values shown) are statistically significant.
Table 4. Adjusted odds ratios in study year, statin-use interaction model
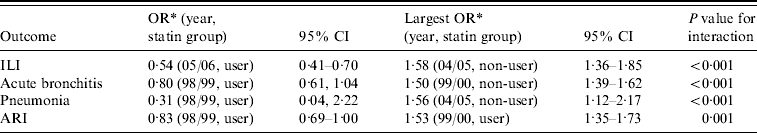
OR, Odds ratio; CI, confidence interval; ILI, influenza-like illness; ARI, acute respiratory infections combined.
* Baseline, 1998–1999, non-user.
DISCUSSION
Our study suggests that, regardless of influenza vaccination status, there was a benefit of statin use to prevent UTI, an increased risk for acute bronchitis, and no effects for ILI, ARI, pneumonia and URTI as a combined group (Table 3). Our findings of no benefit from statins detectable in persons consulting GPs with ARI accord with those recently reported by Smeeth and colleagues based on 9885 outcomes in statin users reported in two groups, ‘pneumonia’ and ‘other serious respiratory infections’ (acute bronchitis and ILI) [Reference Smeeth20]. Our study was based on 7269 outcomes in statin users in four outcome groups with ILI and acute bronchitis in separate groups. The lack of detectable benefit in 17 000 episodes of illness provides very strong evidence that statins are not indicated in the prevention/management of acute respiratory infections including ILI.
The observation that statin use is associated with a reduced risk of UTI enhances the importance and credibility of the negative findings in relation to respiratory infections. Although unexpected, this result supports that reported by Hall and colleagues of a preventive association between statin use and irritable bladder [Reference Hall26], but is contrary to that reported by Smeeth [Reference Smeeth20]. The finding that statin therapy was associated with an increase in the rate of acute bronchitis may potentially result from a selective sensitivity to specific infections or an immunomodulatory activity on localized infection in the upper airways. Gram-positive bacteria and respiratory syncytial virus are recognized as important pathogens in acute bronchitis and the relative importance of these agents in these observations may require further study.
No association was seen between statin use and the incidence of herpes zoster infection. Protection from herpes zoster reactivation is largely dependent on the TH1 T cell immune response and these findings are reassuring that this arm of the immune response is not compromised during statin therapy.
As a general observation on the results for all outcomes, ORs for statin users vs. non-users were less in the influenza vaccinated group compared to the non-vaccinated group. Although individually the differences were not significant, this consistent trend suggests at the least there may be synergism between statin use and influenza vaccination but no suggestion of a negative effect [Reference Packard27]. Regular statin users were defined from a prescription record which included two prescriptions given before 31 December and thus those defined as regular statin users were probably receiving statins when vaccinated as well as at the time of the outcome under consideration. Synergism may be apparent in the immediate immune response to vaccination, or in extending the period of effectiveness of the vaccine. We believe this possibility merits further study.
Data capture for this study was particularly robust. All data items were collected from the primary electronic patient record and no single item depended on memory recall. The GP practices included in the study were part of the RCGP WRS surveillance network in which all morbidities are logged using computer-accessible codes and the same applies to all prescriptions. The continuous patient specific record allowed us to search back over long periods to collect critical items of information. We were able to judge statin status from serial and dated prescription records. The analysis included several winters with influenza epidemics of differing severity and involving differing influenza viruses. The separate analysis in the influenza active period during the millennium winter disclosed no obvious difference between respiratory illness outcomes in this winter relative to others.
There is an extensive literature on confounding in the estimation of influenza vaccine effectiveness [Reference Hak28–Reference Greenland, Lash, Rothman, Greenland and Lash31]. The ‘healthy user’ effect describes selection bias towards persons who although healthy make high use of healthcare services. Some measure of the propensity to consult is used to adjust for this bias. In our study this adjustment was more difficult because persons recruited were not healthy by definition. Accordingly we selected new episodes of illness unrelated to conditions for which there is a possible indication for statin use and used these to create a variable reflecting propensity to consult. Case selection bias may have been influenced by severity of illness which was not directly measured but is partly reflected in the propensity to consult. The study took compliance with treatment into account, by including only those persons showing evidence of regular prescribing and disregarding possible users in the main comparisons. However, confounding from unidentified biases relating to healthcare use can never be excluded.
All the disease endpoints studied were clinically diagnosed and there was no virological confirmation of diagnosis. However the WRS in which this study was based provides the main basis for influenza surveillance in England and the reported incidence of ILI is closely matched with the results of virological investigation in the same network [Reference Fleming and Elliot23]. In most cases the outcomes studied did not involve hospital admission or death and thus it was not focused solely on persons with serious disease. There is no specific reason to think that any effect of statins might differ between viruses: any effect is more likely related to the severity of illness which is implied in the results from studies reported using pneumonia, admissions and deaths as endpoints [Reference Majumdar16–Reference van de Garde19, Reference Fleming21].
Influenza is known to be associated with excess incidence of cardiovascular as well as respiratory hospital admissions and deaths [Reference Fleming, Cross and Pannell32]. Recruitment to this study was predominantly among persons with cardiovascular disease and the findings therefore relate specifically to such persons. If influenza increases cardiac risk and there is good evidence that it does [Reference Fleming, Cross and Pannell32–Reference Verel35], then the use of statins might be expected to reduce adverse cardiac outcomes but it is likely that this beneficial effect is limited to persons with established cardiovascular problems. Increasing evidence in favour of giving statins to all persons with cardiac disease [Reference Ridker36] make the possibility of testing this hypothesis in a randomized treatment trial unlikely in persons with established illness, although we encourage the continued use of observational databases to assess possible benefit in a serious influenza epidemic. If there remains doubt about benefit in severe illness, a randomized placebo-controlled study of over-winter statin use in healthy volunteers could be considered. Since there is no basis for thinking that statins could stop influenza virus infection, a study based on clinical outcome with severity scale endpoints would be required. However, since our findings do not show any convincing benefit for statin use to minimize the respiratory outcomes studied in this population it is most unlikely that a benefit might be observed in a healthy population.
As a summary and general conclusion therefore, these results, based on a large number of observations taken over eight consecutive winter seasons, provide no evidence for the use of statins to limit the incidence of acute respiratory infections (including ILI) presenting in primary care. We believe the results in this study population can be applied to the national population with cardiovascular problems and diabetes and have similar implications for the healthy population. A beneficial effect against serious respiratory illness is not excluded. The findings do not support extensive untargeted prophylactic use in a pandemic which is predominantly respiratory in its clinical impact.
ACKNOWLEDGEMENTS
We were encouraged and inspired to undertake this study by David Fedson. We thank all participating Weekly Returns Service GP practices for allowing us to use their data. We value statistical advice provided from André Charlett and Nick Andrews (Health Protection Agency Centre for Infections) and the help of Professor Paul Moss (Birmingham University) on the interpretation of the immune modulatory effects of statins. This study was funded by Health Protection Agency, England with top-up funding in the form of an educational grant from Pfizer Ltd.
DECLARATION OF INTEREST
D.M.F. and J.V.T. have received funding to attend influenza-related meetings and received consultancy fees from influenza antiviral drug and vaccine manufacturers. D.G. is currently an employee of GlaxoSmithKline.






