INTRODUCTION
Measles mortality fell markedly (>90%) from the 19th century to mid-20th century prior to introduction of measles vaccine or the widespread use of antibiotics for secondary bacterial infections [Reference Engelhardt1]. Many other economic, social and epidemiological changes were occurring at the beginning of the modern age so it is uncertain which changes may have led to the decline in measles mortality. Infection was almost universal prior to the introduction of measles vaccine in the 1960s [Reference Perry and Halsey2]. During military mobilizations in the mid-19th century the USA experienced high adult measles mortality rates particularly in men recruited from rural areas [Reference Woodward3]. As populations increased and transportation spread respiratory pathogens more quickly, measles in adults became rare and the viral infection became a childhood disease sometimes with case-fatality rates up to 100 times what would be expected today, especially in malnourished or otherwise ill children [Reference Wolfson4]. Although public health was rapidly improving a century ago, it is uncertain how such improvements in nutrition and sanitation would have influenced measles mortality rates [Reference Yazdanbakhsh, Kremsner and van Ree5]. Adult measles deaths are an excellent marker for an epidemiologically isolated population. Although adult infections are now rare, they are still thought to be more severe than ordinary childhood measles infections [Reference Cherry, Feigin and Cherry6, Reference Wong and Goetz7]. In many measles mortality studies gender has been shown to be important with female children having higher mortality rates than males [Reference Garenne8]. Pregnancy, crowding, poor nutritional status and cellular immunological impairment have all been noted to contribute to high measles mortality [Reference Burnet9–Reference Ogbuanu12].
Measles can cause tremendous mortality rates across all ages when it first contacts an isolated population as was seen in Pacific Islands such as Fiji in 1875 and Rotuma in 1911 [Reference Corney13, Reference Cliff, Haggett and Smallman-Raynor14]. Besides making the entire community from infants to the elderly ill simultaneously leaving few healthy persons to provide nursing care, first-contact epidemics had particularly pernicious symptoms such as subacute ileocolitis and pneumonia which sometimes resulted in up to one-fifth of the population dying during the epidemic [Reference Corney15–Reference Monif and Hood17]. As most of these epidemics occurred prior to the arrival of medically trained observers, there have been only a few detailed epidemiological studies of first-contact measles epidemics [Reference Shanks18]. In the absence of further historical epidemiological studies, it is difficult to explain the tremendous mortality of first-contact epidemics particularly as mortality rates rapidly decreased in very isolated populations during subsequent epidemics.
We have accessed the earliest measles mortality information available from areas with varying degrees of epidemiological isolation including the Faroe Islands, Iceland, Scotland and Australia/New Zealand with the intent of looking for a transition from first-contact or crisis mortality to modern measles mortality patterns. Even in the earliest records, measles mortality was largely restricted to young children indicating that any mortality changes resulting in age-shifting occurred very rapidly once measles contacted an isolated society. Factors in addition to epidemiological isolation and age that may have influenced mortality rates include changes in nutritional status and in the virulence of the measles virus.
METHODS
Historical epidemiological studies are completely dependent on what records were left by our scientific predecessors such that it is often difficult to report the same measurement over the same time interval. Whenever possible we report actual mortality rates over the same period of time. Cumpston collected the entire Australian experience of public health reports of common infectious diseases in 1927 which is the source of the Australian data presented in this paper [Reference Cumpston19]. Australian states began collecting information at different times (e.g. Victoria from 1858, Tasmania from 1873) so the interval from 1889–1914 is reported for consistency. A similar population not from Australia was sought as a comparison population. Historical New Zealand data were taken from a published source [Reference Maclean20]. Detailed information was available from the Scottish city of Aberdeen from 1883 to 1902 which was used as a less isolated but genetically similar comparison population [Reference Wilson21]. We have reported previously the epidemiology of the 1911 Rotuma measles epidemic and sought a very isolated European population for comparison to first-contact epidemics on Pacific Islands [Reference Shanks18]. In addition to Panum's classical report from the Faroe Islands’ epidemic of 1846, Iceland has good mortality records from 1843 but only all-cause and not measles-specific mortality was reported [Reference Cliff, Haggett and Smallman-Raynor22, Reference Panum23]. Given the historical nature of this information, it was not possible to collect nutritional or non-measles respiratory pathogen information that might have contributed to a larger analysis.
RESULTS
The epidemic progression of measles morbidity and mortality in the six states of Australia is shown in Figure 1. Although all states reported measles in 1893, by the beginning of the 20th century some inter-state differences are seen within the context of local epidemics occurring approximately every 5 years. The percentage of all reported measles deaths within age groups was available for all six states and is shown in Figure 2(a, b). Most measles deaths occurred in young children aged ⩽2 years. The apparent increase at age 5 years is an artifact of the change in scale of age groups from annual to 5-year intervals. Although the measles mortality percentage in adults (age ⩾15 years) varies between the more isolated states of Australia which had fewer international shipping lines a century ago (Tasmania 14·9%, Western Australia 14·6%, Queensland 14·2%) and less isolated states (Victoria 8·5%, New South Wales 9·1%, South Australia 9·2%), there is little difference between the individual states in the age-specific mortality graphs of Figure 2. Measles mortality/100 000 population in Australia in 1883–1884 was reported as 1250 for the <5 years age group, 320 for 5–10 years, 50 for 10–15 years and 190 for >15 years. Measles mortality/100 000 population in Australia in 1893 was reported as 260 for the <5 years age group, 0 for 5–10 years, 0 for 10–15 years and 9 for >15 years [Reference Cumpston19].

Fig. 1. Measles mortality rate/100 000 population in the six states of Australia for the period 1889–1914.

Fig. 2. Measles mortality by age in Australia 1889–1914 expressed as a percentage of all measles deaths in a particular state. (a) Victoria, New South Wales and Queensland; (b) Tasmania, Western Australia and South Australia. The apparent mortality increase at age 5 years is an artifact caused by a change from annual to 5-year intervals.
Often the most appropriate comparison for Australian public health information is New Zealand which shared many similarities with Australia during the colonial era while also having an indigenous population, the Polynesian Maori. Summarized figures from New Zealand public health reports indicate that measles epidemics were seen in 1875, 1882, 1893, 1899, 1903 and 1907 [Reference Maclean20]. During the interval 1875–1907 measles mortality fell from 101 to 11/100 000 European population. From 72% to 87% of all measles deaths in Europeans during 1875–1907 occurred in children aged <5 years whereas from 2% to 18% occurred in those aged >10 years [Reference Maclean20]. During a 1938 epidemic while the European measles death rate was 11/100 000, the comparable Maori rate was 243/100 000 with 84% of the Maori deaths occurring in children aged <5 years.
Aberdeen, Scotland was considered as a genetically similar comparison population with much more respiratory pathogen contact within an urban environment than would have been experienced by children born in Australia or New Zealand at the beginning of the 20th century. Measles epidemics occurred in Aberdeen every 3–5 years as shown in Figure 3. Mortality due to measles during epidemics ranged from 20 to 120 reported deaths per year which amounts to about 50/100 000 total population. Figure 4 demonstrates that most measles deaths in Aberdeen occurred in pre-school children especially those aged ⩽2 years without discernible gender differences.

Fig. 3. Measles morbidity (measles cases/100 000 population) and mortality (number of measles deaths) in Aberdeen, Scotland, 1883–1902.
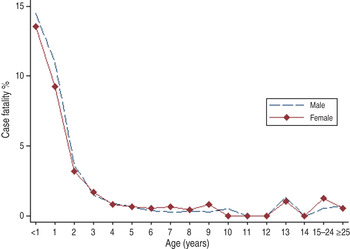
Fig. 4. Age-specific measles case-fatality rate by gender for Aberdeen, Scotland, from 1883 to 1902.
As adult deaths were characteristic of first-contact epidemics and a mortality peak in young adults was observed in Rotuma in 1911, we sought a comparably isolated European population that was new to measles [Reference Shanks18]. Although both the Faroe Islands and Iceland were known to have had previous measles epidemics in 1781 and 1788, respectively, at the time of the 1846 epidemic even many of the oldest inhabitants on both island groups were susceptible to measles [Reference Cliff, Haggett and Smallman-Raynor22]. As the epidemiology of measles in the Faroe Islands and Iceland has been extensively reported previously, only age-specific mortality was examined for comparison purposes [Reference Cliff, Haggett and Smallman-Raynor22–Reference Gunnarsdottir, Briem and Gottfredsson24]. In Iceland 1846, there were 1862 excess deaths in a population of 58 677, giving an estimated measles mortality of 3173/100 000 population. For the subsequent large epidemic of 1882, there were 1648 excess deaths in a population of 73 091, giving an estimated mortality of 2255/100 000 population [Reference Gunnarsdottir, Briem and Gottfredsson24]. If only individuals aged <36 years are counted during the epidemic, assuming universal immunity in the older population, the mortality rate is 3290/100 000 population. On the Faroe Islands a 2·8% case-fatality rate amounting to approximately 2300/100 000 population measles mortality was observed. When the 1846 epidemics on both island groups are compared in Figure 5, Iceland had relatively more measles deaths in older adults (aged 40–60 years) than in the Faroe Islands. The difference in extreme old age is probably a partial artifact when comparing all-cause (Iceland) to measles (Faroe Islands) mortality.
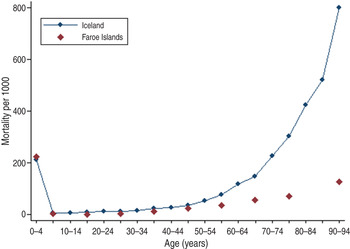
Fig. 5. Mortality in Iceland (all-cause mortality) and the Faroe Islands (measles-specific mortality) during the 1846 measles epidemic.
DISCUSSION
The cause of the extraordinary measles mortality seen during first-contact island epidemics and the decline in measles mortality prior to introduction of vaccine or antibiotics remain poorly understood. Examination of the earliest measles mortality records available did not show a distinct transition from mortality across all age groups to deaths concentrated only in the first two years of life. Potential explanations for extreme measles mortality that may have decreased over time include epidemiological isolation from respiratory pathogens as seen on islands, nutritional status particularly vitamin A and inherent virulence of measles virus.
Societies with rapid circulation of respiratory pathogens soon have very few adults developing measles as children become solidly immune from their first and only measles virus infection. However, even those few adults being infected with measles progressively developed less serious illnesses once they encountered the virus. Men coming from isolated rural areas into the US Army from the US Civil War of the mid-19th century until the Second World War of the 20th century developed measles with progressively decreasing infection rates (32·2 to 4·7 cases/1000 man-years) and mortality (2·0% to 0·004% case fatality) [Reference Cliff, Haggett and Smallman-Raynor22]. During the First World War, Scottish soldiers from isolated highland areas died at high rates (>10%) in recruit camps while most of their urban counterparts had already been infected as children [Reference Kinnear25]. Therefore, isolation alone was not a major mortality risk factor excepting that only by being epidemiologically isolated did an adult escape measles virus during their childhood. It seems more likely that the measles mortality risk associated with isolated populations was due to the rate at which an individual developed immunity to other respiratory pathogens which progressively increased as groups encountered the global pathogen pool from increased outside contacts due to improved transportation. This increased exposure could have decreased secondary bacterial pneumonias following measles. Other possible contributory factors to the fall in measles mortality could be lower viral infectious dose occurring as progressively fewer individuals developed measles during successive epidemics as well as better nursing care when fewer adults were ill during epidemics subsequent to the first introduction [Reference Paunio26, Reference Morens27].
High measles mortality occurs in malnourished children in developing countries indicating the important role of nutritional status in enabling children to survive a severe viral infection [Reference Halsey28]. Malnutrition likely influences measles mortality through limitations in cellular immune responses [Reference Burnet9]. Vitamin A is a particular nutritional component which is important in measles resistance as determined by a variety of vitamin A supplementation studies [Reference Barclay, Foster and Sommer29, Reference Hussey and Klein30]. Could a change in nutritional status explain the historically observed drop in measles mortality? Although childhood nutritional improvements may have contributed to the gradual decline of measles mortality seen just prior to vaccine introduction in the mid-20th century, it is difficult to understand how poor nutrition or its rapid improvement following soon after introduction of measles virus would have accounted for the extreme mortality of first-contact island epidemics and its rapid diminution.
Some RNA viruses show a great range of mortality outcomes such as influenza [Reference Morens and Fauci31]. The intrinsic virulence of measles virus could have varied in a specific population such that mortality rates dropped over time. This explanation seems unlikely as there is no current evidence of measles viruses with differing pathogenicity [Reference Perry and Halsey2]. There are instances where Australian children with a relatively mild rash illness started a measles epidemic leading to great mortality in Aboriginal groups such as in the isolated desert of West Australia [Reference Tooth and Lewis32]. Such examples are more consistent with variation in the host and not in the virus.
Will it ever be possible to state more definitively the cause of extreme measles mortality during first-contact epidemics? Since essentially no un-contacted human populations remain in the 21st century and because it is not possible to reconstruct the details of respiratory pathogen circulation a century after the fact, it seems unlikely that further historical epidemiological studies will be able to be more than suggestive. Although some historical mortality questions have been spectacularly answered by finding tiny amounts of nucleic acid in extant archived human remains, it is very unlikely any sort of biological samples exist which could address the rapid decrease of measles mortality in the pre-vaccine era [Reference Morens and Fauci31].
ACKNOWLEDGEMENTS
The authors thank Dr Rebecca Kippen of the University of Melbourne and Dr Nick Wilson of the University of Otago as well as many medical librarians and archivists who have unselfishly provided data and ideas for this manuscript.
G.D.S. is an employee of the Australian Defence Organization, but there was no specific funding for this particular investigation.
The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defence Force.
DECLARATION OF INTEREST
None.








