INTRODUCTION
Dysentery – defined as invasive diarrhoea with visible blood in one or more stool – is an intestinal inflammation, usually of the colon, resulting in frequent passage of bloody mucoid faeces. Dysentery is often associated with high morbidity and mortality and is found in young children globally, and disproportionately affects those in developing countries [Reference Sansonetti, Van Nhieu and Egile1, Reference Kotloff2]. Additional common symptoms include abdominal cramps, fever, and tenesmus [Reference Hale, Keusch and Baron3] with severity depending upon the disease-causing pathogens [Reference Epple and Zeitz4]. The disease has severe nutritional consequences for children, including protein-losing enteropathy [Reference Hale, Keusch and Baron3] and slower catch-up growth patterns in children for up to 1 year post-infection [Reference Patwari5]. Although Shigella spp. is the single most commonly characterized cause of dysentery [Reference Fischer, Sack and Black6], bloody diarrhoea has also been reported in infections with amoebiasis, Campylobacter enteritis, Salmonella enteritis, enteroinvasive E. coli (EIEC), and enterohaemorrhagic E. coli (EHEC). On average, 10–20% of enteric diseases, and 50% of the dysentery cases in young children can be characterized as shigellosis [Reference Hale, Keusch and Baron3].
Dysentery is perceived as a serious and life-threatening disease in South Asia [Reference Fischer, Sack and Black6]. In rural Bangladesh, dysentery accounts for 39% of all diarrhoea episodes and 62% of diarrhoea-associated deaths, with epidemics causing high rates of morbidity and mortality in all age groups [Reference Kotloff2]. Poor living standards including overcrowding, inadequate sanitation, and poor hand hygiene have been found to be associated with an increased risk of acquiring dysentery [Reference da Paz, de Almeida and Gunther7]. There is a lack of updated knowledge regarding the aetiology and clinical features of dysentery cases in young children. This study aims to illustrate the common bacterial aetiological agents of dysentery, and their associated clinical manifestations in children aged <5 years.
METHODS
Study site
The study was conducted at Kumudini Hospital, in the Mirzapur subdistrict of rural Bangladesh (see Fig. 1). Since 1982, Kumudini Hospital has been operating a separate outpatient and in-patient diarrhoea treatment unit. Nearly 1500 diarrhoea patients (mostly children) report each year to this facility for treatment. Mirzapur subdistrict in Tangail district is located about 60 km north of Dhaka, the capital city, with an area of 374 km2. It has a population of 360 700 whose social attributes have been studied extensively. The study population is composed of male 50%, female 50%; Muslim 85%, and Hindu 15%. The subdistrict has 13 unions (smallest administrative unit) and 219 villages. The surveillance area covers 8/13 unions in Mirzapur (total population 265 626; Demographic surveillance system, December 2011). In the demographic surveillance system area, 11% of the population are children aged <5 years. Average literacy is 33% (male 40%, female 26%). Agriculture is the main occupation while women work mainly in the home. Two main health facilities serve the people of Mirzapur subdistrict: Kumudini Hospital (750 beds) and the subdistrict government health facility (31 beds).
Fig. 1 [colour online]. Map of Bangladesh showing study site and proximity to capital city.
Enrolment, data collection, and laboratory assessment
To facilitate the present hospital-based prospective aetiology study, an active diarrhoeal disease surveillance system was established at Kumudini Hospital. The system recorded data on common enteric pathogens by collecting information on demographic, epidemiological, clinical and nutritional characteristics of patients admitted with diarrhoea. Using structured questionnaires, information on socioeconomic and demographic characteristics, housing and environmental conditions, feeding practices (particularly of infants and young children aged 0–35 months), and use of drugs and fluid therapy in the home was collected from the caregiver, usually the mother. Information on clinical characteristics, prior duration of diarrhoea, nutritional status, duration of hospital stay and treatments received during hospitalization, and outcome of treatments were also recorded. Microbiological assessments of faecal specimens were performed to identify and characterize four common disease-causing pathogens and to determine antimicrobial susceptibility of Shigella, V. cholerae O1, and enterotoxigenic E. coli (ETEC) [Reference Tanaka8, Reference Talukder9], and rotavirus [Reference Rahman10]. The working definition of diarrhoea was ‘3 or more abnormally loose or watery stools during the previous 24 hours’, and for dysentery ‘diarrhoea with visible blood in one or more stool’.
Study population
Data from all children aged <5 years (of either sex, regardless of nutritional status and disease severity), who resided in the Mirzapur demographic surveillance system (DSS) catchment area, and sought care for diarrhoeal disease at Kumudini Hospital, Mirzapur, comprised the cohort for analysis.
Specimen collection and laboratory procedures
Stool specimens were collected from all children aged <5 years presenting with diarrhoea. A single, fresh, whole stool specimen (at least 3 ml or 3 g) was collected from each patient. Written informed consent was obtained from each child's caregiver. A faecal swab was then placed in Cary–Blair medium in a plastic screw-top test tube. Using a Styrofoam container with cold packs, the specimens were transported to the central laboratory in Dhaka within 6 h of collection. All patients who provided informed consent and presented at Kumudini Hospital, Mirzapur with diarrhoea provided a single fresh, whole stool specimen (at least 3 ml or 3 g). Each specimen was aliquoted into three serial containers and submitted to the respective laboratories for routine screening of common enteric pathogens such as ETEC [Reference Qadri11], Vibrio cholerae [Reference Bhuiyan12], Shigella spp. [Reference Talukder13], and rotavirus [Reference Rahman14] applying standard methods. Bacterial isolates were tested for susceptibility to antimicrobials by the disk diffusion test. Shigella species were isolated and identified in the enteric microbiology laboratory by using standard biochemical and microbiological methods (WHO, 1987) [15]. Stool specimens were inoculated on MacConkey and Shigella-Salmonella agar plates and incubated overnight at 37°C. Non-lactose-fermenting colonies characteristically resembling Shigella were inoculated into Kligler's iron agar tube for typical reaction, mannitol fermentation, citrate utilization, urease and indole production, and lysine decarboxylation. Shigella serotypes were confirmed by slide agglutination with polyvalent somatic (O) antigen grouping sera, followed by testing with monovalent antisera for specific serotype identification (Denka Seiken, Japan). In cases where no agglutination occurred with live bacteria, the test was repeated with boiled suspensions of bacteria. Shigella flexneri isolates that were not typable with commercial antisera were typed using a panel of monoclonal antibodies specific for S. flexneri group and type factor antigen [Reference Talukder16].
Data analysis
Data were analysed using SPSS for Windows version 15.2 (SPSS Inc., USA) and Epi Info version 5.6 (Epi Info, CDC, USA). Descriptive statistics were computed with P < 0·05 used as the criterion for statistical significance. A backwards stepwise multiple logistic regression analysis was performed to examine the association between independent variables and the dependent variable (dysentery) with the probability of exclusion at P = 0·10.
Ethics
This present analysis of children aged <5 years was part of a diarrhoeal disease aetiology and burden study which was approved by the Research Review Committee and the Ethical Review Committee of icddr,b in December, 2009.
RESULTS
From January 2010 to December 2011, a total of 2324 children aged <5 years with diarrhoea received treatment at Kumudini Hospital, of which 682 (29%) exhibited symptoms of dysentery (see Fig. 2). Of the dysentery cases, 38% were infants aged 0–11 months, 32% were children aged 12–23 months and the remainder (30%) were aged 24–59 months. The median age was 15 months, and 60% of the study children were male. The median duration and severity of dysentery cases was also greater than other diarrhoea cases. Seventy-three percent of non-dysentery cases lasted less than 72 h, compared to 64% of dysentery cases (P < 0·001). Furthermore, the percentage of cases lasting ⩾7 days was 9% for dysentery and 5% for non-dysentery cases (P < 0·001). Fever, abdominal pain, and straining were also reported significantly more frequently by children with blood in stool. Regarding hygienic practices, 69% of the mothers reported using soap and water for bottom-cleansing procedures of their children post-defecation. A further 60% of households had no fixed place for the disposal of the child's faecal matter. No significant differences were noted between socioeconomic or hygienic explanatory variables and risk of presenting with dysentery vs. non-dysentery illnesses. Children with dysentery were, however, found to be significantly more underweight, stunted and wasted compared to non-dysenteric children. Characteristics of children with and without dysentery are summarized in Table 1.
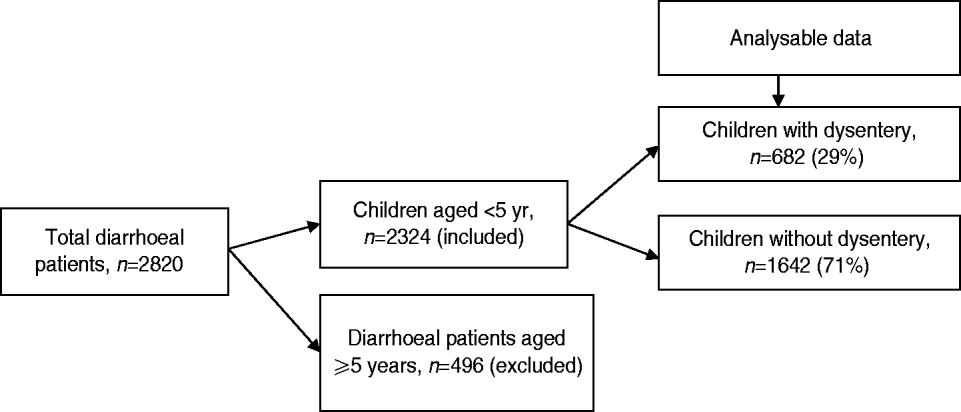
Fig. 2. Flowchart showing breakdown of study participants.
Table 1. Characteristics of children with dysentery, Mirzapur, rural Bangladesh
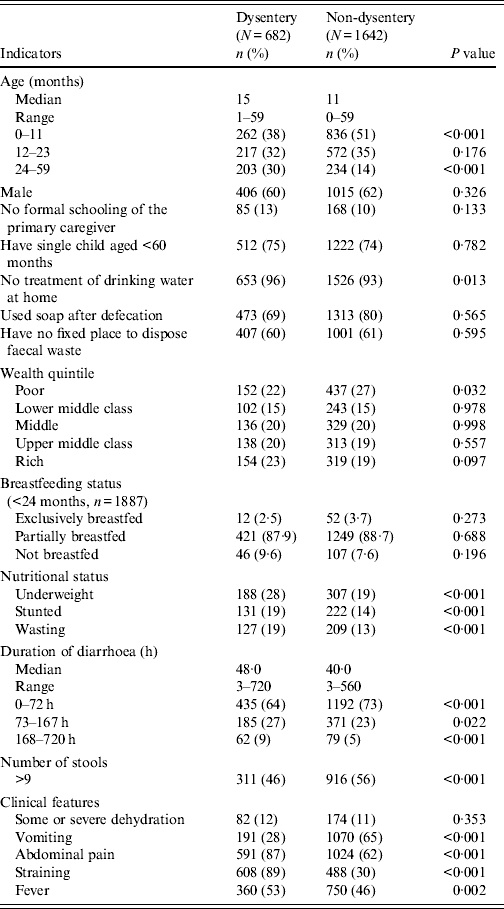
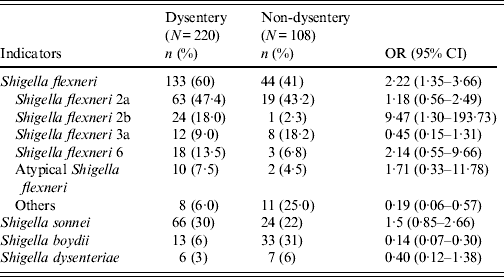
Table 2 compares aetiological agents in dysenteric and non-dysenteric children. Of the 682 dysentery cases; 32% had confirmed shigellosis, 4% had rotavirus, 3% had ETEC, and 2% Vibrio cholerae O1. Additionally, 3% were found to have had mixed pathogens in their stool. Moreover, dysentery of indeterminate aetiological cause accounted for 61% of cases, compared to 47% of the other diarrhoea cases. Of 220 confirmed shigellosis cases aged <5 years, 60% were of S. flexneri serogroup, 30% S. sonnei, 6% S. boydii, and 3% S. dysenteriae. Of the S. flexneri isolates, 47% were S. flexneri 2a, followed by S. flexneri 2b (18%), S. flexneri 6 (14%), S. flexneri 3a (9%), atypical S. flexneri (8%) and others (6%) (Table 3).
Table 2. Aetiological agents with bloody diarrhoea in children aged <5 years, Mirzapur, rural Bangladesh
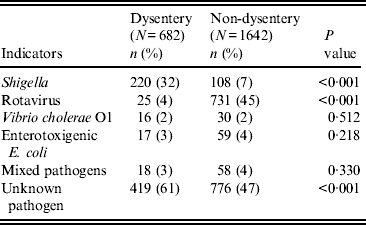
Table 3. Subgroups of Shigella-positive diarrhoea for children aged <5 years, Mirzapur, rural Bangladesh
OR, Odds ratio; CI, confidence interval.
Atypical Shigella flexneri (S. flexneri 1c, S. flexneri 4, S. flexneri 4a, S. flexneri 5b); others [S. flexneri 3b, S. flexneri X, S. flexneri Y, S. flexneri 1a, S. flexneri 1b, S. flexneri poly(+)].
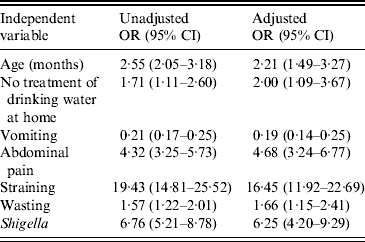
Significant associations were found between dysentery and: child's age (24–59 months), no treatment of drinking water at home, vomiting, abdominal pain, straining, wasting, and presence of Shigella in stool specimens. These associations maintained significance when incorporated into a multivariate model after controlling for the potential confounders such as gender, years of schooling of mother/primary caregiver, breastfeeding status, number of children aged <5 years in the household, and type of toilet facility used (Table 4).
Table 4. Association between blood in stool and other characteristics of study children, Mirzapur, rural Bangladesh
OR, Odds ratio; CI, confidence interval.
Outcome variables: age of the child (1 = 24–59 months, 0 = 0–23 months), treatment of drinking water at home, vomiting, abdominal pain, straining, weight-for-height status, and presence of Shigella in stool.
Main exposure: Presence of blood in stool, yes vs. no (presence of blood was the reference category).
Adjusted factors: gender, years of schooling of mother/primary caregiver, breastfeeding status, number of children aged <5 years old in household, and toilet facilities.
DISCUSSION
The study produced some interesting results. First, the cohort was found to be composed of 60% male and 40% female patients: a finding which echoes that of other studies [Reference Ahmed, Billoo and Murtaza17, Reference Farshad18]. This may be attributable to gender differentials in health-seeking behaviour for male and female children as has been reported in regions throughout the world, including neighbouring West Bengal, India [Reference Pandey19]. Second, the results indicate that the average duration of dysentery cases was greater than that of non-dysentery cases; with some episodes of dysentery lasting for up to 30 days. This may be due to the real and perceived seriousness of bloody diarrhoea [Reference Kotloff2], which can result in more rapid seeking of medical attention [Reference Slade and Schwartz20]. Third, incidence rates of dysentery were found to increase gradually during the first 2 years of life. The associated consequences have been documented in a previous study [Reference Guerrero21]. The significant changes in incidence rates of dysentery between the ages of 2 and 5 years, could be explained by susceptibility determined by the lack of consumption of breast milk [Reference Bode and Jantscher-Krenn22]. Weaning results in decreasing concentrations of maternal antibodies as well as introduction to non-sterile food and drink [Reference Slade and Schwartz20, Reference Bode and Jantscher-Krenn22].
Of the dysenteric children, one third were confirmed shigellosis cases; 4% had rotavirus followed by ETEC (3%), and Vibrio cholerae O1 (2%). Three percent were found to have mixed infections, which may account for some of the dysentery symptoms in the proportion of children infected with non-classical dysentery-associated pathogens (i.e. cholera and rotavirus). Globally, the majority of Shigella isolates originate from developing countries, where S. flexneri is the dominant strain. Although Talukder et al. [Reference Talukder9], reported S. flexineri 2b as the dominant subtype while we observed S. flexineri 2a as the predominant subtype like others [Reference Khan23]. Diarrhoea of an indeterminate cause was significantly more common in children with dysentery than those without. Similar findings have been revealed by a recent study in Pakistan [Reference Soofi24] and a systematic review determining the infectious aetiology of diarrhoea [Reference Fischer, Sack and Black6]. Researchers have indicated that most diarrhoeal episodes begin due to a specific enteric pathogen. The results identified that many patients have co-infection with multiple pathogens while no pathogen could be isolated from others [Reference Fischer, Sack and Black6]. Previous studies reported EIEC, EHEC, Campylobacter, Salmonella, and Entamoeba histolytica to be responsible for dysentery, particularly prolonged dysentery which could have been due to amoebiasis; however, those pathogens were not detected in the present study [Reference Petri25, Reference Pfeiffer, DuPont and Ochoa26]. It has been reported that children with more severe disease often have a higher bacterial load in their stool [Reference Vu27].
The study sought to quantify the effects of several socioeconomic indicators on the relative increased risk of dysentery compared to non-dysenteric diarrhoea in children presenting with diarrhoea. Characteristics including income, drinking water treatment, post-defecation hand hygiene, faecal disposal and primary caregivers' or parental education have been shown to affect rates of diarrhoea [Reference Birmingham28–Reference Victora32]. No significant differences were identified in socioeconomic characteristics between the families of dysenteric vs. non-dysenteric children (Table 1). In contrast to socioeconomic characteristics, nutritional indicators were found to be significant. Upon presenting to a health facility, children with dysentery were found to be significantly more underweight, stunted and wasted than children suffering from non-dysenteric diarrhoea. This finding of poorer nutritional outcomes in dysenteric children supports results from elsewhere [Reference Birmingham28]. One study indicated greater detrimental effect of shigellosis on growth of children [Reference Hale, Keusch and Baron3]. Because, pre-illness data were not collected, poorer relative nutritional statuses could be either a predisposing factor to dysentery or a consequence of the illness.
The poor nutritional statuses of children with dysentery could be explained by the immunological and physiological effects of the illness. Young children have been found to have a less effective immune response to Shigella infections than adults [Reference Sansonetti, Van Nhieu and Egile1, Reference Kotloff2]. The defining symptoms of dysentery, bloody diarrhoea are caused by breaching of the intestinal epithelial barrier, due to the invasion and destruction of the intestinal mucosa [Reference Sansonetti, Van Nhieu and Egile1, Reference Vu27]. The combination of mucosal damage and associated inflammatory response can cause elevated temperature, intestinal muscular spasms, swelling of intestinal mucosa, abdominal pain and tenderness, vomiting and dehydration. Such patho-physiological effects can result in anorexia, impaired nutrient absorption and excess protein, mineral and water loss [Reference Hale, Keusch and Baron3]. The combination of the physiological and immunological effects with the longer-than-average duration of dysenteric illnesses could explain the adverse nutritional consequences observed in the study.
One limitation of this study stems from the issues surrounding hospital-based observational studies. A selection bias is likely to have occurred, as those who sought care at the hospital are likely to differ in many respects from those also affected by diarrhoea or dysentery but who did not seek care at the hospital. Moreover, a large number of pathogens like EIEC, EHEC, Campylobacter, Salmonella, and protozoas were not tested which is another limitation of the study that could have led to a high proportion of dysentery cases of unknown aetiology.
The study reiterates findings from the general literature, that dysentery is a common disease, with Shigella spp. accounting for majority of infections in children under five. Although Shigella (S. flexneri) represented the most commonly identified causative organism, the majority of cases had an indeterminate cause. Even though understanding of the determinants of dysentery has evolved, dysentery remains a strong component of diarrhoeal disease – in itself one of the biggest threats to child health in developing countries. The high proportion of undiagnosed dysentery cases remains a concern. This represents an additional limitation of the study, in that it sought to isolate only four common enteropathogens: Shigella, ETEC, rotavirus, and Vibrio cholerae O1. The mapping of the cause of the remaining 60% of dysentery cases should remain the highest priority. Without knowing what diseases are responsible for the majority of illness, it is not possible to design effective interventions.
However, given our understanding of faecal-orally transmitted pathogens it is likely that most cases can be attributed to poor access to sanitation, water and inadequate hygienic behaviours post-defecation. The results of the study underscore the importance of community-based interventions that aim to interrupt faecal–oral transmission pathways, the most effective of which remains sanitation interventions. There is a greater need for further prospective studies on young children in Bangladesh to better understand the burden of dysentery, map the infectious aetiology and their associated risk factors in diverse geographical settings as well as sociocultural context.
ACKNOWLEDGEMENTS
The research protocol was funded by Swedish International Development Cooperation Agency (Sida), grant number MD-0020; and GR-00599. The icddr,b gratefully acknowledges the commitment of Sida to its research efforts. We thank the Medical Director of Kumudini Hospital for his generous support to the research team. Finally, we thank all staff members who actively participated in this study.
DECLARATION OF INTEREST
None.








