INTRODUCTION
Extended-spectrum cephalosporins, such as ceftriaxone, cefotaxime, and ceftazidime, were approved for human therapy in the early to mid-1980s and are commonly used to treat infections caused by enterobacteria. Shortly after their release, clinical isolates of enterobacteria appeared that were able to produce extended-spectrum β-lactamases (ESBLs) [Reference Bauernfeind and Horl1–Reference Knothe3]. These enzymes hydrolysed β-lactam antibiotics, thereby mediating resistance to these agents [Reference Bush4]. Today, infections by ESBL-producing enterobacteria are a serious clinical problem in Taiwan as they are often related to outbreaks in intensive care units (ICUs). Previous reports from Taiwan and other countries have indicated that the most predominant ESBL-producing species are Klebsiella pneumoniae and Escherichia coli [Reference Pfaller and Segreti5–Reference Yu, Chuang and Walther-Rasmussen7] with the prevalence rate of these strains being relatively higher in Taiwan, ranging from 8·5% to 29·8% in K. pneumoniae and from 1·5% to 16·7% in E. coli, respectively [Reference Yu, Chuang and Walther-Rasmussen7].
At Chang Gung Memorial Hospital (CGMH), we have screened isolates for ESBL production according to the guidelines described by the Clinical and Laboratory Standards Institute (CLSI) since September 2000. Data from our previous report indicated that in the first year there was a relatively high prevalence of ESBL-producing enterobacteria (E. coli, 4·6%; K. pneumoniae, 15·6%) [Reference Wu6]. We have continued to monitor trends of these bacteria up to 2007. Studies of the genetic relatedness of ESBL genes in ICU isolates were also undertaken in three separate time periods.
METHODS
Setting and laboratory-based surveillance
CGMH is a university-affiliated, tertiary hospital with 4000 beds located in northern Taiwan. There are 26 ICUs providing critical care to patients; the other 73 general wards are included in the inpatient department. A case of nosocomial infection was defined according to the updated criteria of the Centers for Disease Control and Prevention, USA [Reference Garner8].
Bacterial isolates
In total, 84458 E. coli and 31633 K. pneumoniae non-duplicate clinical isolates were identified by standard methods and their susceptibilities to the following antimicrobial agents were determined by a disk diffusion method according to CLSI guidelines [9]: amikacin, ampicillin, aztreonam, cefazolin, ceftazidime, ceftriaxone, cefuroxime, ciprofloxacin, gentamicin, imipenem, piperacillin, and trimethoprim/sulphamethoxazole. Duplicate isolates from the same body site of a patient within 30 days were excluded from statistical analysis. ESBL production was confirmed by a disk diffusion method using cefotaxime (30 μg) and ceftazidime (30 μg) in combination with 10 μg clavulanate as recommended [9].
A total of 568 non-duplicate ESBL-producing isolates of E. coli (n=273) and K. pneumoniae (n=295) were collected from ICU patients over three separate periods (I: August 2002–December 2003; II: January–June 2005; III: October–December 2007) and subjected to molecular investigation.
Molecular typing
Isolates were typed by infrequent-restriction-site PCR as previously described [Reference Su10] and banding patterns were compared visually. Isolates showing >4-band difference in profile were categorized as different genotypes; those with identical band patterns or that differed by ⩽3 bands were considered indistinguishable or closely related, respectively, and designated as the same genotype.
Multiplex PCR and SHV melting curve mutation detection (MCMD) system
The multiplex PCR and SHV MCMD system was used to determine the ESBL genotypes of isolates [Reference Chia11]. Briefly, the multiplex PCR identified bla SHV, bla CTX-M-3-like, and bla CTX-M-14-like genes and a modified SHV MCMD method was used to distinguish six prevalent bla SHV genes (bla SHV-1, bla SHV-2, bla SHV-2a, bla SHV-5, bla SHV-11, bla SHV-12) in Taiwan. An 819-bp product of the bla SHV was amplified over 50 cycles and two hybridization probes were used concomitantly in a single reaction tube in a LightCycler. Fluorescence signals were plotted automatically in real time vs. temperature (T) to produce melting curves for mutations at position 35 (F3 vs. T) and positions 238 and 240 (F2 vs. T) of the SHV gene. Melting curves were then converted into melting peaks by plotting the negative derivative of fluorescence vs. T (−dF2/dT vs. T and –dF3/dT vs. T). Isolates positive for CTX-M genes were subjected to further PCR and DNA sequencing analysis to identify the specific CTX-M types.
PCR amplification and DNA sequencing
During period I, six pairs of previously described primers were used to detect bla TEM [Reference Mabilat, Goussard, Persing, Smith, Tenover and White12], bla SHV [Reference Rasheed13], and four major groups of bla CTX-M genes, including bla CTX-M-2-like, bla CTX-M-3-like, bla CTX-M-8-like, and bla CTX-M-14-like [Reference Chia11, Reference Ma14, Reference Pitout, Hossain and Hanson15] in a collection of 149 E. coli and 177 K. pneumoniae isolates. As no other ESBLs except SHV, CTX-M-3, and CTX-M-14 types were identified during this period, the multiplex PCR and SHV MCMD system described above was adopted in the following two periods to determine the major ESBL types followed by direct PCR and sequencing of the identified SHV and CTX-M genes using three pairs of previously described primers [Reference Chia11, Reference Rasheed13, Reference Ma14]. All PCR products were purified using Microcon® PCR centrifugal filter devices (Millipore, USA) and sequences determined using an ABI 3100 Avant Genetic Analyzer (PerkinElmer, Applied Biosystems, USA). Homologous sequences in the GenBank database were searched for using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST/).
Statistical analysis
The χ2 test and Student's t test were used to determine the significance of differences. A difference was considered statistically significant with a P value <0·05.
RESULTS
Prevalence of ESBL-producing isolates
A total of 7007 E. coli and 5729 K. pneumoniae isolates were identified as ESBL producers. The majority of isolates were from urine (56·3%; E. coli, 64·2%; K. pneumoniae, 46·7%), followed by the respiratory tract (16·8%; E. coli, 10·7%; K. pneumoniae, 24·2%) and sterile sites (13·4%; E. coli, 13·0%; K. pneumoniae, 13·8%). A significant increase was found in the proportions of urinary ESBL-producing E. coli isolates (from 59·5% in 2001 to 66·3% in 2007, P<0·01), and of these isolates, a significant increase was evident for patients from the outpatient department (OPD) (from 22·1% in 2001 to 31·6% in 2007, P<0·005). A similar situation was observed in the proportion of respiratory K. pneumoniae isolates (from 17·0% in 2001 to 26·2% in 2007, P<0·00005); however, the increase was only found in respiratory isolates from patients in the IPD (from 32·0% in 2001 to 51·1% in 2007, P<0·001).
Figure 1 a shows that there was a significant increase in the number of ESBL-producing isolates of both species over the study period (P<0·0001). The prevalence rate also increased significantly from 4·8% in 2001 to 10·0% in 2007 (average 8·3%) in E. coli and from 15·0% to 23·4% (average 18·1%) in K. pneumoniae; these rates were both highest in isolates from ICUs (reaching 24·3% in 2006 for E. coli and 36·9% in 2007 for K. pneumoniae), and lowest in those from OPD where there was a slight increase for E. coli and no change for K. pneumoniae.
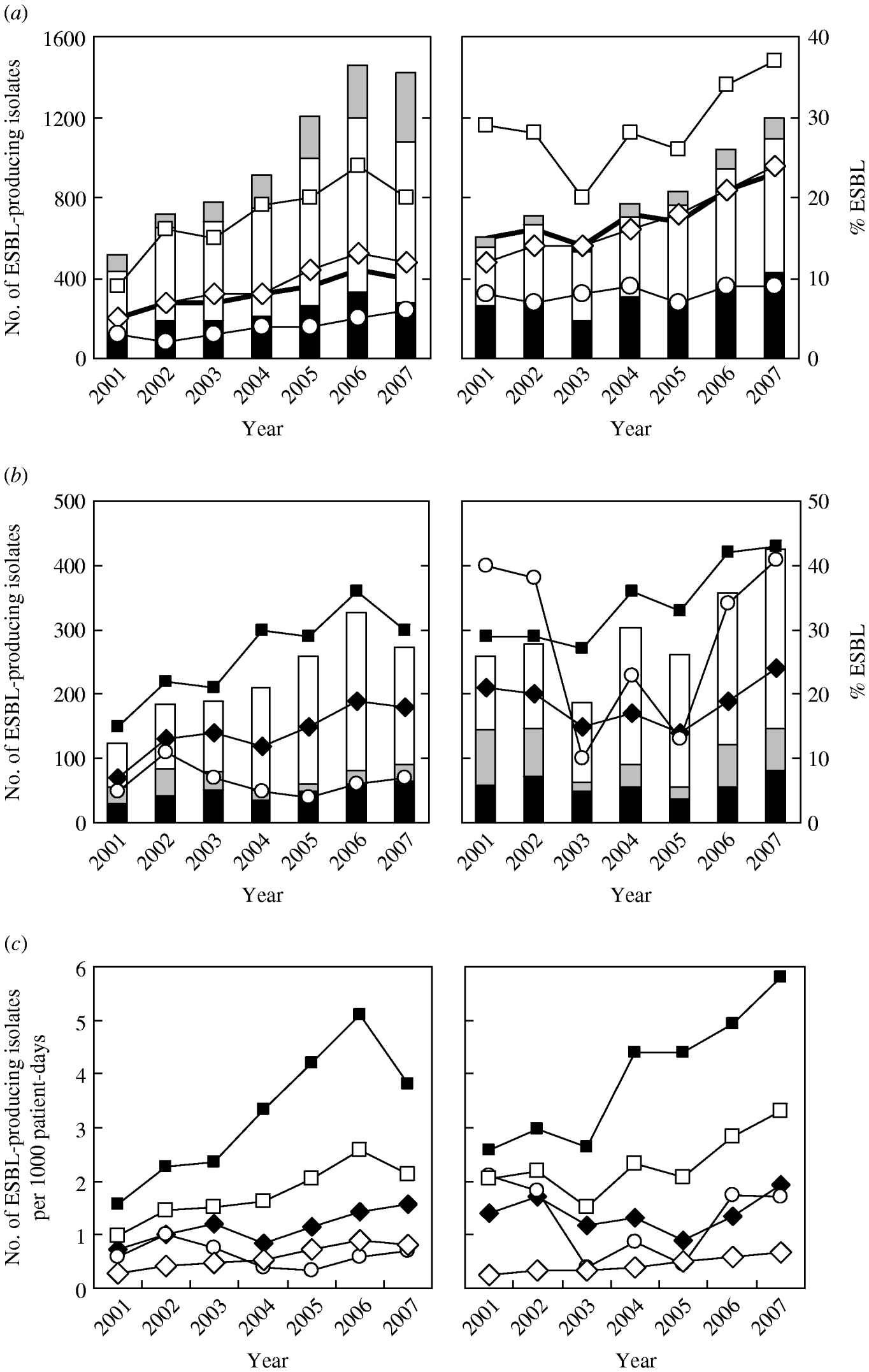
Fig. 1. Secular trends for the annual number (histograms) and prevalence/infection density (lines) of extended-spectrum β-lactamase (ESBL)-producing E. coli (left panels) and K. pneumoniae (right panels) isolated between 2001 and 2007 at Chang Gung Memorial Hospital. Panel (a) demonstrates the variation observed in isolates derived from intensive care units (ICUs) (–□– and black histograms), inpatient department (–◊– and white histograms), and outpatient department (–○– and grey histograms). Panel (b) demonstrates the variation observed in isolates derived from medical ICUs (–▪– and white histograms), surgical ICUs (–◆– and black histograms), and paediatric ICUs (–○– and grey histograms). Panel (c) demonstrates the infection density per 1000 patient-days in isolates derived from inpatient department (–◊–) and ICUs (–□–), including medical ICUs (–▪–), surgical ICUs (–◆–), and paediatric ICUs (–○–).
Analysis of the distribution of ESBL-producing isolates in different ICUs (Fig. 1 b) showed that the most significant increase (P≪0·0001) both annual numbers and prevalence rates occurred in medical ICUs (MICUs), with a peak prevalence rate of 35·9% (in 2006) for E. coli and 42·7% (in 2007) for K. pneumoniae isolates. The second largest group was from surgical ICUs (SICUs) although the increase in the prevalence in E. coli was more marked than in K. pneumoniae. There was a fall in the prevalence of ESBL-producing isolates in paediatric patients during 2003 and 2005 for K. pneumoniae, although that of E. coli remained constant at a low level during the period.
The infection density rates per 1000 patient-days for ESBL-producing isolates were much higher in MICUs in particular, than those in the IPD (Fig. 1 c) but were relatively similar between SICUs and paediatric ICUs (PICUs). No difference in the trend of infection density between the two bacteria was evident, except that a sharp decrease occurred in E. coli in 2007, compared to the continuous increase in K. pneumoniae. The proportion of ESBL-producing nosocomial isolates was relatively constant in E. coli [IPD, 18·9% (range 16·5–23·5%); ICUs, 26·0% (range 23·8–26·6%); MICUs, 28·0%; SICUs, 23·8%; PICUs, 19·6%)], and for K. pneumoniae, the proportion in IPDs reduced significantly from 22·4% during 2001–2004 to 17·0% during 2005–2007 (P<0·0005), but remained constant in ICUs [25·2% (range 22·8–29·0%); MICUs, 26·9%; SICUs, 21·5%; PICUs, 22·6%].
ESBL types
Sixteen ESBL types (9 SHV and 7 CTX-M) were identified (Table 1). CTX-M-14 was the most prevalent followed by CTX-M-3 and SHV-12. CTX-M-14 was also the major ESBL type in E. coli isolates, whereas SHV-12 was more prevalent in K. pneumoniae. Some rare or novel ESBL types were identified, but E. coli was more likely to be associated with CTX-M-type enzymes (CTX-M-13, CTX-M-24, CTX-M-27), while K. pneumoniae was characterized by SHV-2, SHV-26, SHV-27, SHV-28, SHV-31, and SHV-61. Significant differences were found in the proportion of various ESBL types in the PICUs with CTX-M-3-producing isolates (E. coli, P<0·05; K. pneumoniae, P<0·0001) being the most prevalent but fewer in CTX-M-14- (E. coli, P<0·000005; K. pneumoniae, P<0·0001) and SHV-12-producing isolates (K. pneumoniae, P< 0·005) (Table 1).
Table 1. Distribution of various extended-spectrum β-lactamase (ESBL)-producing isolates of E. coli and K. pneumoniae in intensive care units (ICUs)
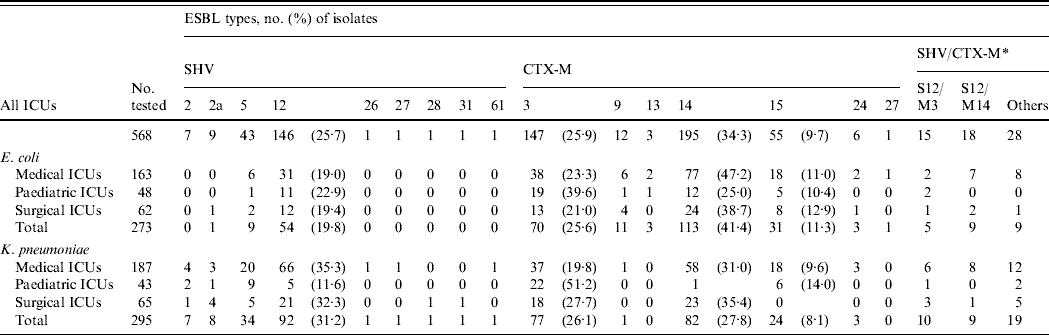
S, SHV ESBLs; M, CTX-M ESBLs.
* The numbers of isolates producing dual ESBLs in the area are indicated. Other sporadic combinations are S2/M3, S2/M14, S2a/M3, S2a/M14, S2a/M15, S5/M3, S5/M14, S12/M13, S12/M15, S26/M14, S27/M14, S28/M3, S31/M14, S61/M15, M3/M14, and M14/M15.
There was no significant difference in the changing trends of ESBL types in different ICUs over the three periods. The overall trends of the major types (Fig. 2) showed that for E. coli, the proportion of CTX-M producers increased gradually while SHV producers declined (Fig. 2 a). Although CTX-M-14 was the most frequent type overall in E. coli isolates, the proportion of CTX-M-3 producers was very similar to that of CTX-M-14 producers during the first study period (April 2002–December 2003). Subsequently, the proportion of CTX-M-3-producing isolates reduced sharply and was accompanied by a sudden increase of CTX-M-15-producing isolates (Fig. 2 a). Replacement of bacterial populations from predominant CTX-M-3 producers to CTX-M-14 producers in the second and third study periods as well as the reverse trends of CTX-M-3- and CTX-M-15-producing isolates was also found in K. pneumoniae (Fig. 2 b). The reduction of CTX-M-3-producing E. coli isolates was most significant in PICUs and SICUs where none of such isolates were recovered during the second and third study periods. Compared to the other ESBL populations, the proportion of respiratory isolates was higher (32·7%; E. coli, 35·7%; K. pneumoniae, 29·6%), and lower or similar for urinary CTX-M-15-producing isolates (47·3%; E. coli, 46·4%; K. pneumoniae, 48·1%). Co-production of two different ESBLs by a single isolate was not unusual (E. coli, 8·4%; K. pneumoniae, 12·9%). Other than five isolates that produced two CTX-M-type enzymes, the others all produced various combinations of SHV/CTX-M ESBLs, with SHV-12/CTX-M-14 and SHV-12/CTX-M-3 being the most frequent combinations (Table 1).
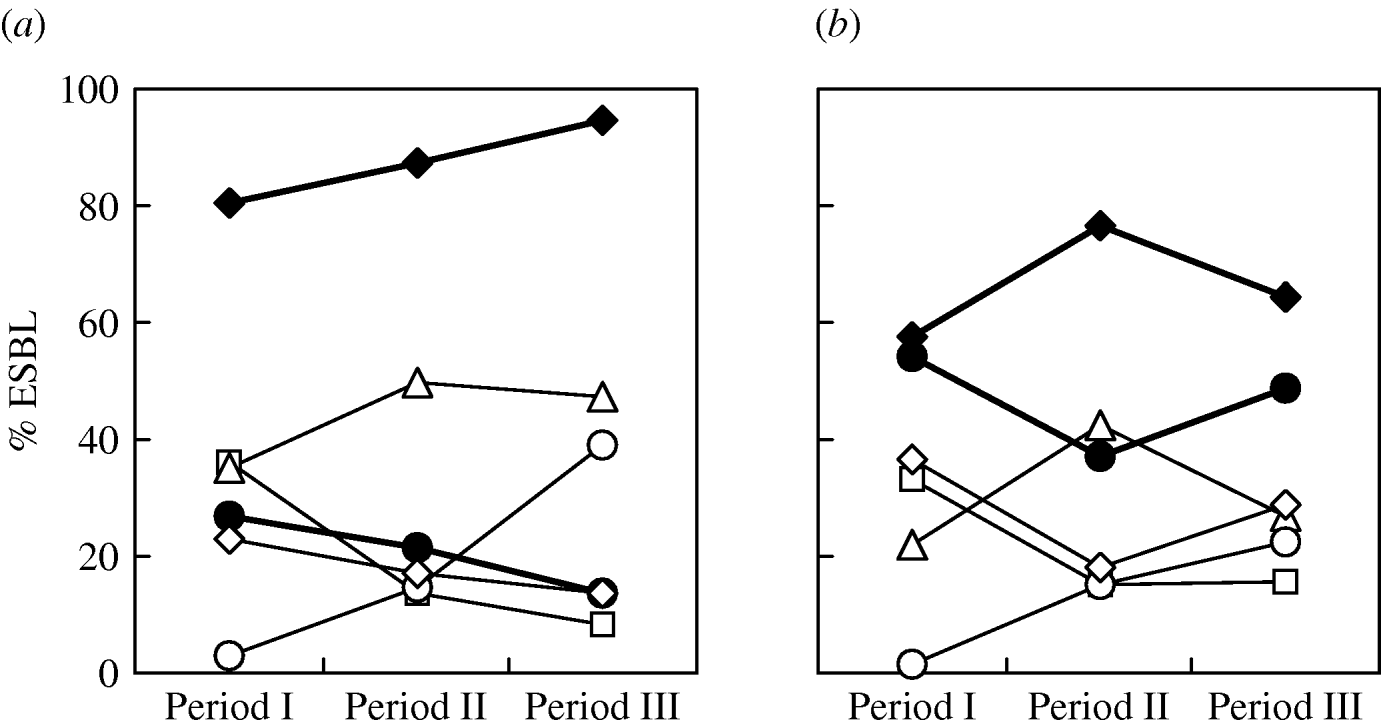
Fig. 2. Prevalence of various extended-spectrum β-lactamases (ESBLs) (CTX-M, –◆–; CTX-M-3, –□–; CTX-M-14, –▵–; CTX-M-15, –○–; SHV, –•–; SHV-12, –◊–) in clinical isolates of E. coli (a) and K. pneumoniae (b) obtained in period I (August 2002–December 2003), period II (January 2005–June 2005), and period III (October 2007–December 2007).
Genotypes
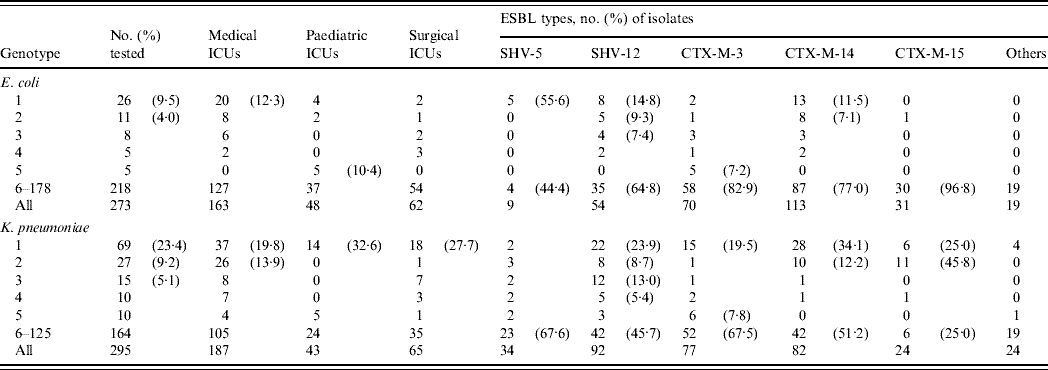
Both species were highly diverse with 178 and 125 genotypes being defined for ESBL-producing isolates of E. coli and K. pneumoniae, respectively (Table 2). Multiple isolates were found only in genotypes 1–36 in E. coli and in genotypes 1–29 in K. pneumoniae. In E. coli, the largest cluster, genotype 1, was also the most predominant in MICU isolates, but it was sporadic in other ICUs. Similarly, isolates of other prevalent genotypes (2, 3, 4) were distributed in various ICUs, but genotype 5 was restricted to PICU isolates. Less genotype diversity was evident in K. pneumoniae isolates since >50% of the total fell into only ten genotypes. Of these, genotype 1 was the largest cluster and was also the most prevalent in all three ICUs. By contrast, the majority of genotype 2 isolates, the second largest group, were found in MICUs. For both species, isolates belonging to genotypes 1 and 2 could be found throughout the study periods.
Table 2. Distribution of various genotypes in the extended-spectrum β-lactamase (ESBL)-producing isolates of E. coli and K. pneumoniae in different intensive care units (ICUs)
Correlation between ESBL types and genotypes
Genotypes 1 and 2 isolates of E. coli were significantly associated with the production of CTX-M-14 in MICU isolates, as 18/21 isolates were recovered from MICUs over the three study periods (P<0·0005). However, other significant correlations were revealed between SHV-5-producing isolates and genotype 1 (P<0·001), and between CTX-M-3-producing isolates and genotype 5 (P<0·001) (Table 2). The five genotype 5/CTX-M-3-producing isolates were all recovered from PICUs within 3 months during the first study period, while the other five genotype 1/SHV-5-producing isolates were all recovered from MICUs during the first (n=2) and second (n=3) study periods.
Significant correlations of genotypes and ESBLs were also found between isolates of K. pneumoniae: CTX-M-15 and genotype 2 (P<0·000001), and SHV-12 and genotype 3 (P<0·0001) (Table 2). The 12 genotype 3/SHV-12-producing isolates were all recovered during the first study period from MICUs (n=6) and SICUs (n=6) whereas the 11 genotype 2/CTX-M-15 producers were isolated from patients in MICUs during the second (n=5) and third (n=6) study periods. There were some differences between the distribution of isolates producing different ESBLs in the ICUs: CTX-M-14-producing isolates were found only in MICUs and SICUs throughout the study periods (P<0·001), 14/15 CTX-M-3-producing isolates were recovered in the first study period and seven of them were from PICUs, and likewise five of the six CTX-M-15-producing isolates were found in PICUs during the second (n=4) and third (n=1) study periods (P<0·005). Furthermore, five genotype 2 isolates recovered in MICUs within 7 months during the first study period were found to produce SHV-12 and CTX-M-14 concomitantly.
DISCUSSION
The data reported herein are the most up-to-date as well as the largest series of ESBL prevalence studies in Taiwan [Reference Hirakata2, Reference Wu6, Reference Yu, Chuang and Walther-Rasmussen7, Reference Jean16]. In contrast to our earlier findings from this hospital [Reference Wu6], we have demonstrated a significant and sustained increase and substantial variations in ESBL production in E. coli and K. pneumoniae isolates. A report from eight countries in the Asia-Pacific region revealed that, during 1998–2002, the prevalence rates of ESBL-producing isolates varied from 0·5% to 24·5% (overall 5·9%) in E. coli and 3·7% to 35·6% (overall 17·3%) in K. pneumoniae [Reference Hirakata2]. Another report from the region focusing on intra-abdominal infections indicated that the prevalence of ESBL-producing isolates in E. coli and Klebsiella spp. was 19·6% and 22·9%, respectively, in 2004 [Reference Rossi17]. They also showed that, although only 1 year apart, the ESBL rates appeared to increase slightly in 2004 [Reference Paterson18]. Another report from Korea further demonstrated ESBL production in 10·2% of E. coli and 22·4% of K. pneumoniae isolates in 2005 [Reference Ko19]. Considered together these data indicate a generally growing trend of ESBL-producing population in the Asia/Pacific region. However, due to the difference in the participating countries or institutions in different studies, direct comparison of rates may not be feasible. In contrast, through the current longitudinal study, we have been able to demonstrate that the prevalence rates of ESBL producers in Taiwan were relatively similar to those of the neighbouring countries reported previously during the corresponding years, and that the prevalence of the ESBL producers indeed has continuously grown in the past few years.
A recent report from ICUs in Taiwan indicated that in 2005, the prevalence of ESBL production in E. coli and K. pneumoniae was 14% and 26%, respectively [Reference Jean16]. Our findings for ICUs during the same period showed a slightly higher rate in E. coli but similar in K. pneumoniae. As a result, the longitudinal surveillance reported here suggests that such a significant increase in the ESBL-producing bacteria may be not merely a local observation but may also be reflective of the Asia-Pacific region. Furthermore, the current study also clearly shows that the significant increase of ESBL-producing isolates was more likely observed in the hospital (ICUs and IPD) than in the community (OPD). It is known that the heavy use of antibiotics in hospitals may more easily induce the occurrence of resistant bacteria [Reference Hsueh, Chen and Luh20]. Hence, more prudent use of antimicrobial agents may be required to curtail the continuous increase of ESBL-producing populations.
Some ESBL types, such as TEM-, VEB-, and CTX-M-types other than CTX-M-3-like and CTX-M-14-like ESBLs, were only identified with low prevalence in Asian countries, including Taiwan [Reference Livermore21]. We noted a similar situation in the first sampling period of this study, and as a consequence these rare ESBLs were not further investigated in the following two periods. In contrast, previous studies indicated that in Taiwan, the most prevalent ESBL types were SHV-5, SHV-12, CTX-M-3, and CTX-M-14 [Reference Yu, Chuang and Walther-Rasmussen7]. Compared to our earlier study [Reference Wu6], there has been a marked expansion (from 5 to 16 types) in the number of ESBL types circulating in the country. A significant and continuous increase in the proportion of CTX-M-producing isolates was clearly shown in E. coli from 69·2% previously [Reference Wu6] to 94·4% in the third period of this study; the figures for K. pneumoniae fluctuated between 58% and 77% in both studies [Reference Wu6]. On the other hand, a significant decline of SHV-producing isolates was also clear in E. coli from 35·9% previously [Reference Wu6] to 13·9% in our study, and for K. pneumoniae the proportion decreased from 60·8% previously [Reference Wu6] to between 37% and 54% in our study. Our results are consistent with those reported from European countries where the predominant ESBL types have changed dramatically from SHV and TEM types in the 1990s to CTX-M types at present [Reference Livermore22]. Only in North America do SHV and TEM ESBLs still predominate [Reference Bush23].
A recent study from Thailand reported the almost universal prevalence (>99%) of bla CTX-M in ESBL-producing E. coli and K. pneumoniae [Reference Kiratisin24]. Dissemination of the bla CTX-M genes was postulated to be probably due to the potential mobilization ability of some insertion sequence (IS) elements, especially ISEcp1, which was present in the upstream region of bla CTX-M in most isolates [Reference Kiratisin24]. Moreover, the 3′-end of the ISEcp1 insertion sequences was suggested to probably provide intact -35 and -10 promoter regions for the expression of the CTX-M enzymes [Reference Kiratisin24–Reference Woodford26]. The insertion of ISEcp1 upstream of the bla CTX-M genes has become a common phenomenon and has been reported frequently in many countries or institutions [Reference Gonullu27–Reference Zong30], including our hospital [Reference Liu31]. Investigation of recent isolates has revealed the widespread existence of ISEcp1 in the CTX-M-producing strains (L.-H. Su, unpublished data). Accordingly, before any strategies are implemented to control the increase, the occurrence in the near future of the high prevalence or endemicity of such resistant populations as observed in Thailand may be expected in Taiwan.
The continuous increase of CTX-M ESBLs was mostly found in the increase of isolates that produced CTX-M-14 between the first two periods of the current study. However, during the same period, a dramatic reverse change was also evident between the proportions of isolates that produced CTX-M-3 (decreasing) and CTX-M-15 (increasing). Although isolates that produced CTX-M-3 and CTX-M-14 both reduced in the third period, the increase of CTX-M-15-producing isolates continued and contributed to the overall increase in the proportion of CTX-M-producing isolates. This finding is in accord with reports from many European countries [Reference Livermore22, Reference Coque, Baquero and Canton32] as well as from North America [Reference Lewis33, Reference Zhanel34] and Australia [Reference Zong30] where a rapid spread of CTX-M-15-producing isolates has been observed. In India, where CTX-M-15 was first reported in 2001 [Reference Karim25], the enzyme has now become the most predominant ESBL [Reference Hawkey35].
CTX-M-15-producing isolates in Taiwan were first reported in 2004 [Reference Yu36]. The gene encoding region of CTX-M-15 is only a single nucleotide substitution difference from CTX-M-3, leading to the higher ceftazidime-hydrolysing activity [Reference Karim25]. This modified activity appears to have a great impact on the superior prevalence of CTX-M-15 over CTX-M-3 [Reference Yu36]. However, the mechanisms responsible for its significant increase in our study appear to be different between the two bacterial species. Previous reports from Canada [Reference Pitout37] and UK [Reference Woodford26] indicated that some epidemic CTX-M-15-producing strains were responsible for the wide dissemination of such resistance in E. coli. This contrasts with our finding of diverse genotypes in CTX-M-15-producing E. coli isolates, suggesting that the prevalence of such resistant isolates may be due to the dissemination of resistance plasmids or other mobile elements carrying the bla CTX-M-15 gene. Nevertheless, it is only recently that a pandemic urovirulent E. coli clone O25-ST131, as determined by serotyping and multilocus sequence typing, has been detected in many European countries as well as Canada, India, Japan, Korea, and Lebanon [Reference Coque, Baquero and Canton32, Reference Lau38–Reference Suzuki40]. Whether or not our CTX-M-15-producing E. coli isolates also belong to, or will be influenced by, this pandemic clone warrants a further investigation. On the other hand, over 70% of the CTX-M-15-producing K. pneumoniae isolates here were found in isolates of genotype groups 1 and 2, indicating that other than transmission of bla CTX-M-15-containing plasmids, clonal spread may also play an important role in the further expansion of the bla CTX-M-15 gene in K. pneumoniae. There was no apparent difference in the infection types associated with the two genotypes compared to other CTX-M-15-producing isolates.
Three rare ESBL types, SHV-31, SHV-61, and CTX-M-27, found here have not been reported previously in Taiwan. SHV-31 differs from SHV-1 by two amino-acid substitutions and has only been reported in an isolate of K. pneumoniae in The Netherlands [Reference Mazzariol41]. SHV-61 was also only found in Portugal before this survey (M. M. M. Canica and N. R. Mendonca, GenBank AJ866284, http://www.lahey.org). On the other hand, CTX-M-27 is very similar (one amino- acid difference) to the prevalent CTX-M-14, and although it was not found previously in Taiwan, recent reports have indicated its widespread occurrence in many countries, including Thailand [Reference Kiratisin24], Spain [Reference Romero42], China [Reference Liu43], Canada [Reference Pitout37], and Egypt [Reference Mohamed Al-Agamy, El-Din Ashour and Wiegand44]. Whether or not isolates that produced this ESBL will propagate to be as prevalent as those observed nowadays in CTX-M-15 producers merits careful monitoring.
In conclusion, this large-scale, longitudinal surveillance for ESBL-producing isolates provides an excellent model to track changes of prevalence rates, ESBL types and epidemic strains over a 7-year period. The frequent use of β-lactam antibiotics may promote the growth in the prevalence of ESBL-producing isolates as well as the variation of ESBL types either from mutations of existent ESBL genes or transmissions of resistance genes or plasmids from other bacteria. The presence of some prevalent strains in both species also suggests that cross-transmission of these resistant pathogens may have occurred in patients. Therefore to effectively control the continuous increase of these resistant bacteria, not only should antibiotic prescribing be more strictly supervised, but also the compliance of infection control measures needs to be reinforced. The burst of CTX-M-15-producing isolates should be more cautiously monitored in Taiwan for its possible widespread dissemination as has already been observed in other countries.
ACKNOWLEDGEMENTS
This work was supported by grants (CMRPG360861 and CMRPG361232) from the Chang Gung Memorial Hospital.
DECLARATION OF INTEREST
None.






